Accelerate Perfusion Media Development and Predict Large-Scale Processes using a Single-use, Automated Microbioreactor
Read more about
- A single-use, automated microbioreactor accelerates perfusion media screening and optimization
- Compatibility with both dynamic and steady state perfusion cell cultures
- Prediction of bench-scale bioreactor performance: Reliability of results
- Accelerating development of perfusion media
- A wide range of applications will benefit from a highly predictive, automated microbioreactor
A single-use, automated microbioreactor accelerates perfusion media screening and optimization
Perfusion is an approach for process intensification with the goal of reducing manufacturing footprint and costs while at the same time increasing productivity and product quality. However, development and optimization of cell culture media for perfusion processes can be complex and time-consuming, requiring a variety of models and scales ranging in size from 1 mL to 3 L. Small-scale models such as 96-deep well plates and spin tubes have high throughput capabilities and are well-suited for screening purposes, but do not have controlled environments nor automated perfusion capabilities. At the other end of the spectrum, single-use benchtop bioreactors have tight control over the culture environment but are not high throughput and require larger media volumes; as such, they are best suited for confirmation runs at the later stages of the development process
This page describes the use of the Mobius® Breez Microbioreactor to rapidly screen and optimize media formulations for perfusion processes and to predict the performance of larger scale bioreactors as an alternative to traditional small-scale models, e.g., spin tubes and deep-well plates. This is one of several applications of the versatile microbioreactor, which also accelerates and streamlines cell line development and cell metabolism studies.

Figure 1.Components of the Mobius® Breez Microbioreactor perfusion platform
The Mobius® Breez Microbioreactor is a small-scale solution for intensified perfusion development, able to reliably predict large-scale performance attributes (Figure 1). The system has a high level of automation and offers functionalities of a perfusion bioreactor system, including the cell retention filter. The four independently controlled bioreactors with a 2 mL working volume each can be run simultaneously, enabling accelerated development timelines and increased efficiency in perfusion screening during cell line development, media screening and optimization, as well as early process development steps.
Advantages offered by the Mobius® Breez Microbioreactor during this case study of perfusion media screening and optimization include:
- Accelerated perfusion media development timelines by up to 50%
- High quality data comparable to larger-scale bioreactors
- Replacement of small- and mid-scale steps (i.e., 3 L bioreactors) used in the conventional media development workflow
- Reduction in media and reagent costs by up to 1000x
- Reduced number of confirmation runs needed during media development
Compatibility with both dynamic and steady state perfusion cell cultures
In dynamic perfusion, the viable cell density (VCD) is allowed to grow as high as possible without any cell bleed control; in the steady state mode, VCD is maintained at a defined level by bleeding cells from the bioreactor. While dynamic perfusion can reach very high VCD and titer level within a short culture time, run duration is limited by cell viability, waste accumulation and potential drops in product quality over time. Steady-state perfusion offers the benefits of stable process, productivity, and product quality, but long culture times, complex set-up and control as well as potential product loss due to cell bleeding can be an issue. A suitable approach needs to be selected on a case-by-case basis taking the individual cell line and downstream process into consideration. Case study results confirm that the Mobius® Breez Microbioreactor can be used for both dynamic and steady state perfusion (Figure 2). Data were generated with a CHOZN® CHO K1 cell line in EX-CELL® Advanced HD Perfusion Medium in the 2 mL working volume microbioreactor at 37°C, pH 7.0, and 40% dissolved oxygen (DO).
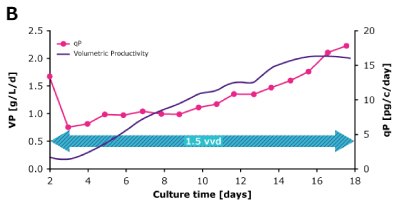
In a dynamic perfusion culture, a high viable cell density of 150 x 106 cells/mL was achieved using the Mobius® Breez Microbioreactor at a perfusion rate of 1.5 vessel volumes per day (VVD), corresponding to a cell-specific perfusion rate (CSPR) of <10 pL/cell/day. CSPR is the amount of media perfused through a single cell per day and is calculated by dividing the perfusion rate by the viable cell density, which represents the medium exchange rate needed to sustain a given cell clone in a perfusion process. Volumetric productivity (VP) and cell-specific productivity (qP) increased over time; VP reached approximately 2 g/L/day, while the qP reached approximately 20 pg/c/day.
In a steady state perfusion culture, VCD was maintained at 50 x 106 cells/mL at 1.5 and 1.0 VVD (CSPR ~18-20 pL/cell/day). At both perfusion rates, the VCD and viability were maintained at constant levels. Both VP and qP were also maintained at a stable level during the steady state perfusion culture.
Dynamic

Steady State
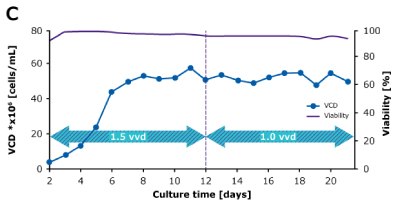
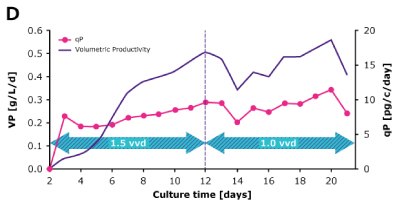
Figure 2. Dynamic (top) and steady state (bottom) perfusion performed using the Mobius® Breez Microbioreactor, measuring viable cell density (VCD), viability, volumetric productivity (VP), and cell-specific productivity (qP) over cell culture time.
Prediction of bench-scale bioreactor performance: Reliability of results
When developing a perfusion medium, it is important that it is high-performing and can be easily transferred to manufacturing scale. Small-scale models that can reliably predict the performance at a larger scale are useful tools that help to improve process efficiency and reduce development timelines. Case studies confirm that the Mobius® Breez Microbioreactor is predictive of the results that would be obtained from larger bioreactors for VCD, cell viability and productivity. Compared to the more traditional approach using spin tubes, the Mobius® Breez Microbioreactor showed superior predictability.
When the Mobius® Breez Microbioreactor was compared with a 3 L glass bioreactor operated at different perfusion rates using a CHOZN® GS cell line cultured in EX-CELL® Advanced HD perfusion medium, cell growth was similar for both systems and at all perfusion rates (Figure 3A). Viability was higher in the Mobius® Breez Microbioreactor system, which was most likely due to low shear stress resulting from bubble-free operation. In the case of the specific productivity (Figure 3B), there was no significant difference between the two bioreactor scales, suggesting that the Mobius® Breez Microbioreactor is highly predictive of the performance of bench-scale bioreactors.
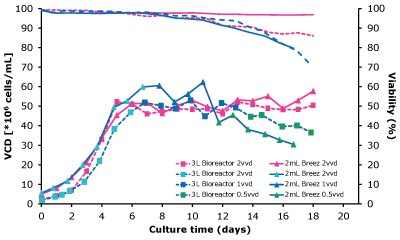
A)Cell growth

B)Specific productivity
Figure 3 A and B. Comparison of cell growth (A) and specific productivity (B) of a CHOZN® GS cell line when cultured in a Mobius® Breez Microbioreactor or a 3L glass bioreactor.
In a second step, the traditional small-scale approach using spin tubes, the Mobius® Breez Microbioreactor, and a bench-scale glass bioreactor were compared. Table 1 shows that process parameters for the microbioreactor and bench-scale system are quite similar indicating that the microbioreactor can be used to predict performance at larger scales. Spin tubes lack a controlled environment and can only run mock perfusion cultures, where media is only batch-exchanged one or several times daily, and as such, this approach is labor-intensive and not highly predictive of larger scale systems.
It is important to note that the Mobius® Breez Microbioreactor can be inoculated at 20 x 106 cells/mL, which is a very high cell density and approximately 10x higher than that enabled by a bench-scale glass bioreactor (see Table 1). With such a high cell inoculation density, the VCD setpoint can be reached much earlier in comparison to a bench-scale bioreactor.
Table 2 summarizes the titer and specific productivity for CHOZN® cell lines grown in a 3 L glass bioreactor, the Mobius® Breez Microbioreactor, and spin tubes. Results from the Mobius® Breez Microbioreactor and the glass bioreactor are very similar with differences of 10% or less, while the comparison of results from the glass bioreactor and spin tubes displayed higher discrepancies in terms of titer. The data suggest that the microbioreactor is highly comparable to both scaled-out and larger scale bioreactors, in terms of specific productivity.
Finally, the predictability of results was compared using different cell lines, different media, and glass bioreactors of different sizes. Three CHOZN® GS and one CHO K1 cell line were cultured in microbioreactors and either 1, 3, or 15 L glass bioreactors using Cellvento® 4CHO-X Expansion Medium and EX-CELL® Advanced HD Perfusion Medium. Results for the CHOZN® GS CHO cell lines in the Mobius® Breez Microbioreactor showed comparable productivity to those achieved using glass bioreactors regardless of medium or bioreactor volume (Figure 4). Results for the CHOZN® CHO K1 cell line showed slightly lower productivity in the glass bioreactor but still a good comparability between both scales.
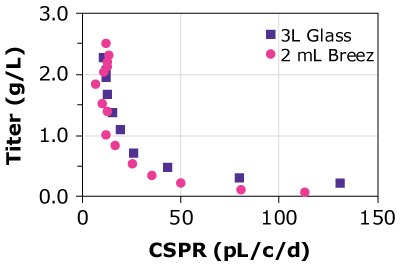
A)CHOZN A - HD
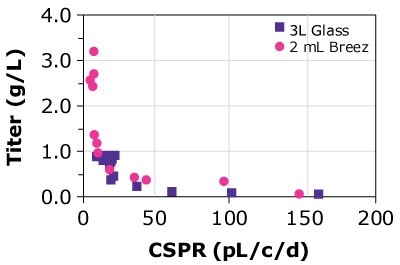
B)CHOZN B - 4CHOX
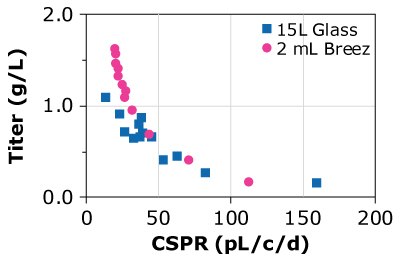
C)CHOZN C - HD
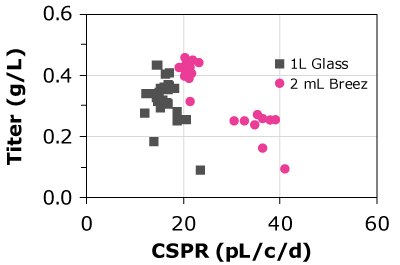
D)CHOK1 - HD
Figure 4. Cell-specific perfusion rate (CSPR) and protein titers for different CHO cell lines grown in either the Mobius® Breez Microbioreactor or a bench-scale glass bioreactors of varying sizes using two different media.
Accelerating development of perfusion media
CSPR is the amount of media perfused through a single cell per day and it is calculated by dividing the perfusion rate by the viable cell density. While running a perfusion culture, a lower CSPR is normally preferred due to lower media consumption, but the optimal CSPR is clone-dependent and requires a comprehensive investigation to optimize media formulations. A case study was performed to assess the suitability of the Mobius® Breez Microbioreactor for screening for both minimum CSPR (CSPRmin) and critical CSPR (CSPRcrit), two important parameters when developing and optimizing perfusion media formulations.
Screening for CSPRmin
In the case of dynamic perfusions, because the VCD is allowed to grow to the maximum level, a CSPRmin can be reached at a constant perfusion rate (Figure 5A). For steady state perfusion, the CSPRmin is not achievable since the cells are allowed to continue growing through a controlled cell bleed, while the VCD is maintained at a constant level well below the maximum VCD (Figure 5B).
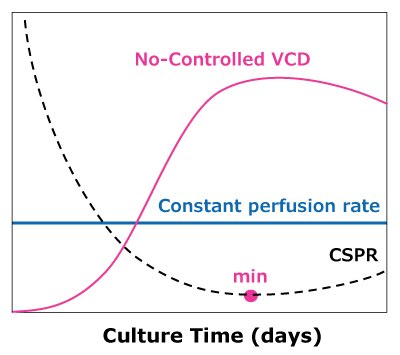
A)Dynamic perfusion
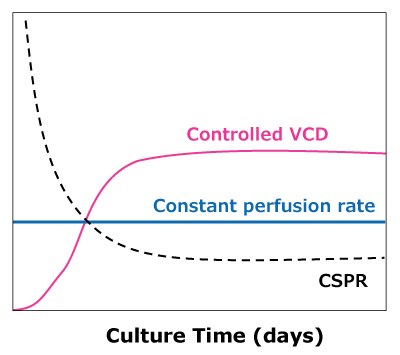
B)Steady state perfusion
Figure 5. Illustration of viable cell density (VCD) and cell-specific perfusion rate (CSPR) profiles in dynamic (A) and steady state (B) perfusion, showing how a minimum CSPR can be reached using dynamic perfusion.
Figure 6 and Table 3 compare the VCD and CSPR for different cell lines; cells were allowed to reach a maximum VCD and the CSPR was allowed to reach the minimum level. Results of the study showed that CSPRmin was highly clone dependent.
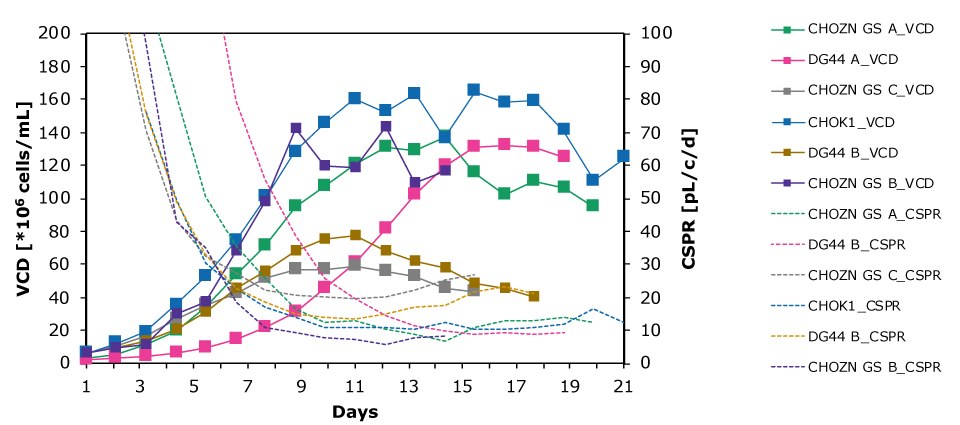
Figure 6.The Mobius® Breez Microbioreactor can be used to screen for the minimum cell-specific perfusion rate (CSPRmin), which is highly clone dependent.
Screening for CSPRcrit
Understanding the limitation of a cell-media combination is crucial to designing new media formulations. The Mobius® Breez Microbioreactor can also be used to screen for CSPRcrit, which is defined as the lowest CSPR at which the steady state criteria are maintained. If the perfusion culture is operated below this level, the steady state will be lost. Limitations indicated by CSPRcrit can be determined using a push-to-low approach, meaning that the perfusion rate is reduced in a stepwise manner until the steady state is lost. The CSPRcrit is defined as the CSPR of the penultimate step, assuming that the steady state is lost at the last stage.
In this study, multiple CHO clones of varying phenotypes including CHOZN® CHO K1, CHO-S, DG44, and CHOZN® GS were evaluated using a range of perfusion rates in Cellvento® 4CHO-X Expansion Medium. A series of Mobius® Breez Microbioreactors was used to complete screening for the critical CSPR of the perfusion culture of the clones over a 20 to 74-day duration. Neither simulated perfusion nor bench-scale reactors were efficient for this screening study.
As shown in Figure 7 and Table 4, CSPRcrit is also highly clone dependent and reinforces the need to balance nutrient needs when developing perfusion media to effectively support different cell lines.
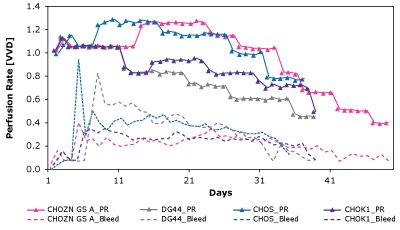
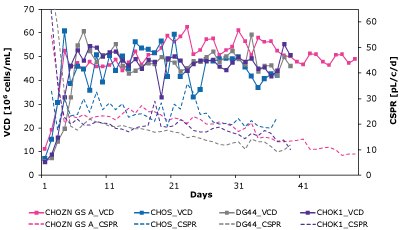
Figure 7. The Mobius® Breez Microbioreactor can be used to screen for critical cell-specific perfusion rate (CSPRcrit), which is highly clone dependent.
A wide range of applications will benefit from a highly predictive, automated microbioreactor
The presented case studies confirm that the Mobius® Breez Microbioreactor is a feasible option for an accelerated cell culture media development process due to the good correlation of data to bench-scale bioreactors. In addition, high-quality, high throughput data acquired using the Mobius® Breez Microbioreactor, in combination with multivariate data analysis tools, can produce powerful predictive modeling necessary for perfusion media development where traditional mock perfusion models fail.
With true perfusion capabilities, the Mobius® Breez Microbioreactor can play a key role in the development of perfusion processes and beyond, positively impacting the upstream workflow through a variety of applications:
Cell line and media development
- Clone ranking
- Cell line stability/performance
- Media evaluation and development
- Raw material variations
- Reagent and additive evaluations
Cell metabolism studies
- Dynamic vs steady state perfusion
- Cell density impact on product quality and economics
- Starvation and metabolic stresses
- Carbon-13 tracing and metabolomics
Process prediction
- Temperature, pH, dissolved oxygen (DO), dissolved carbon dioxide (dCO2) shifts and control bands
- Perfusion and bleed studies
- CSPR determination
- Reproduction of other important conditions at smaller scales to accelerate optimization
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?
