High-Throughput 3D Cell Culture Using TrueGel3D® HTS Hydrogel Plates
3D Cell Culture Models
3D cell culture models have emerged as alternatives to animal models and have led to the creation of more predictive in vitro cell models for drug discovery and compound screening. Traditional 3D cell cultures rely on encapsulating cells in undefined Engelbreth-Holm-Swarm (EHS) sarcoma derived basement membrane (BME) extracts such as Matrigel® matrix (E1270). These hydrogels contain a heterogeneous mixture of both growth factors (TGFβ, EGF, IGF, etc.) and ECM proteins (laminins, collagens, entactin, etc.) at elevated non-physiological concentrations. Due to the extreme temperature sensitive nature of the material, these hydrogels require laborious sample preparation steps and precautions which make them unsuitable for high-throughput drug screening applications. Recently, more defined synthetic hydrogels have been developed for 3D cultures which are more consistent and animal-free but still require users to premix cells and hydrogels together to encapsulate.
TrueGel3D® HTS Hydrogel Plates
The TrueGel3D® HTS Hydrogel Plate is a ready-to-use solution to easily establish 3D cell cultures using fully synthetic hydrogels in a simple and automation-compatible manner. The 96-well polystyrene glass-bottom plates contain pre-casted synthetic functionalized PEG based hydrogels. These innovative hydrogels contain gradually increasing crosslinking densities throughout the well. Users can proceed directly with seeding cells without any hydrogel preparation or encapsulation steps. After cell seeding, cells will gradually infiltrate the hydrogel and establish a 3D cell culture environment within days. Furthermore, this newly engineered hydrogel surface gives the user the possibility to sequentially establish co-culture systems by seeding different cell populations at different time-points in the same hydrogel well.
Features and Benefits:
- No Hydrogel Assembly Step Required: Ready for cell seeding.
- Fully Synthetic: Lot-to-lot consistency and control over culture conditions. No animal derived products.
- Sequential Seeding: Easily co-culture various cell populations at different time points.
- Designed for Imaging: Glass bottom 96-well black polystyrene plates. Precast hydrogel is optically clear.
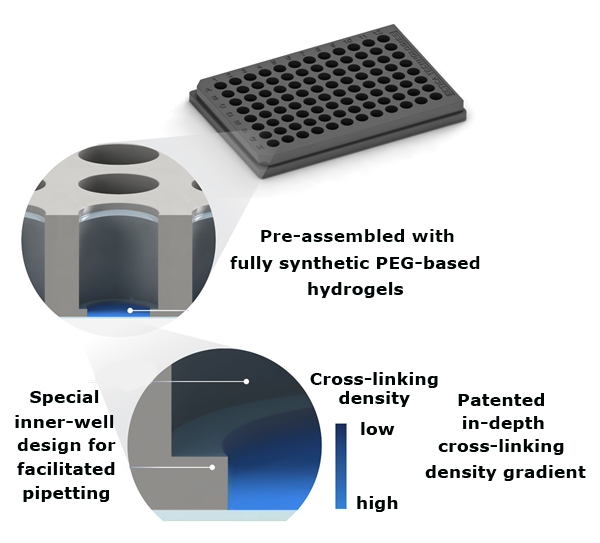
Figure 1.TrueGel3D® HTS Hydrogel Plate technology overview. Synthetic functionalized PEG hydrogels are precast into glass bottom black polystyrene 96-well imaging plates.1
Hydrogel Chemistry
This synthetic hydrogel is constituted of enzymatically crosslinked multi-arm poly(ethylene glycol) (PEG) - peptide bioconjugates. Multi-arm PEG monomers are terminally conjugated to peptides containing metalloprotease-cleavable motives to support cell-mediated degradation, cell adhesion motives and enzymatic crosslinking sites. The polymeric network is polymerized using an enzymatic transglutamination reaction. A unique patented hydrogel surface, consisting of an “in-depth” crosslinking density gradient, is created during the hydrogel casting process.
How to Use TrueGel3D® HTS Hydrogel Plates
Step 1: Remove storage buffer.
Step 2: Add cells and media and incubate overnight.
Step 3: Establish co-culture (Optional): Add secondary cells and incubate overnight. Note: More detailed step-by-step instructions can be found in the online TrueGel3D® HTS Hydrogel Plate user guide.

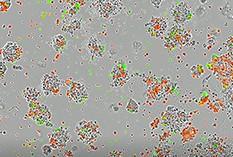
Figure 2. T-cell mediated killing of tumor cells.A) EBC-1 human lung squamous carcinoma cells were incubated with activated (+Cpd)/non-activated CD3+/CD23+ T-cells (red) in TrueGel3D® HTS Hydrogels. Cytotoxicity was analyzed using a green nucleic acid live/dead cell dye after a 72-hour incubation. Co-cultures of EBC-1 cells with Cpd activated T-cells displayed enhanced (2X) dose-dependent tumor cell killing overtime.

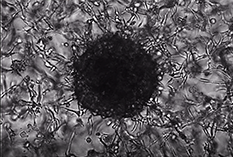
Figure 3. Tumor spheroid formation.10,000 MIA-PaCa-2 human pancreatic carcinoma cells were seeded and cultured in TrueGel3D® HTS Hydrogels in DMEM, 10% FBS, 1% Pen/Strep over 14 days. Cells were stained for cytokeratin 19 (CK19) (red) after 14 days. Cells show robust tumor spheroid formation after 14 days. Various carcinoma lines and primary tumor cells can be cultured to observe tumor spheroid formation and tumor cell invasion of the surrounding matrix.
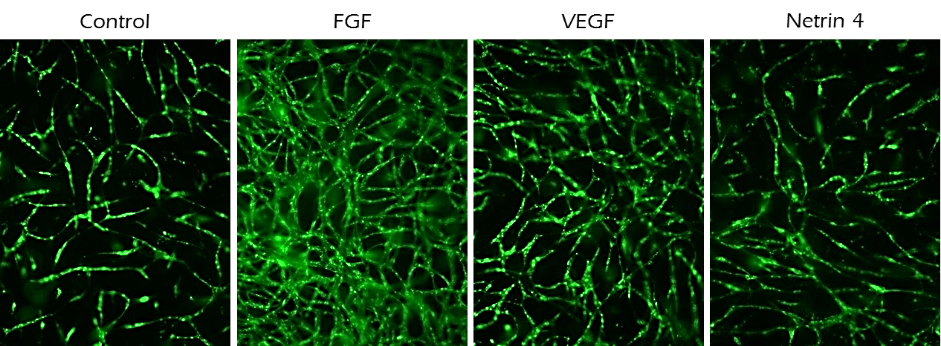
Figure 4. Angiogenesis assays.10,000 GFP labeled endothelial cells (HUVECs) were seeded in TrueGel3D® HTS Hydrogels with Human MSCs supporting cells (not fluorescently labelled) in 200 µL growth medium (70% MEM-alpha, 20% EGM-2, 10% FBS, 1% Pen/Strep and 10 ng/mL FGF). FGF, VEGF or Netrin-4 was added to media on the following day to evaluate their angiogenic potential. Angiogenesis was analyzed at day 10 by imaging and quantifying endothelial cell tubule length. FGF and VEGF increased angiogenesis while Netrin-4 inhibited angiogenesis.
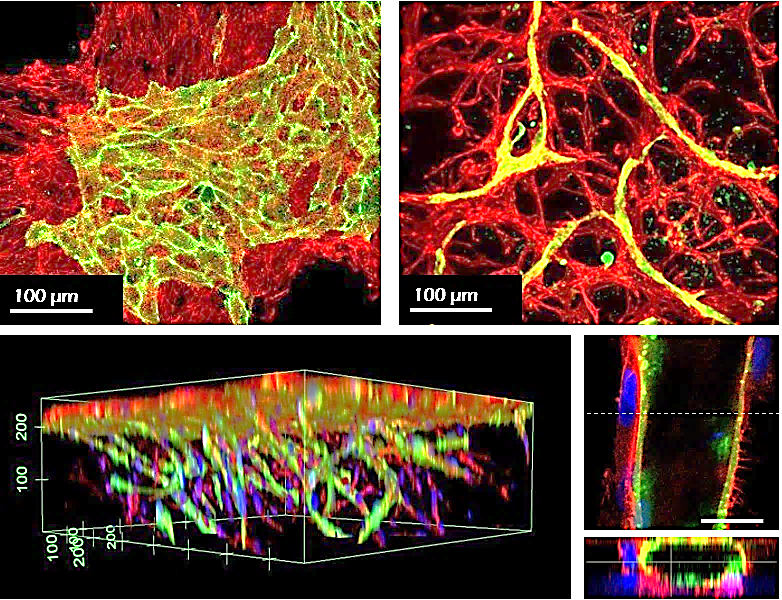
Figure 5. Mesenchymal stem cell/endothelial cell co-culture. Human MSCs (red) and HUVECs (green) were co-cultured in conventional hydrogels (left) or TrueGel3D® HTS Hydrogels (right/bottom) for 7 days. The cells on the conventional hydrogel remained on the surfaces and segregated, whereas the cells deposited in TrueGel3D® HTS hydrogel invaded the gel and self-organized in cord-like vascularized 3D structures.

Figure 6. Tumor cell microenvironment.10,000 RFP labeled fibroblasts were seeded in TrueGel3D® HTS Hydrogels in 200 µL of invasion medium (MEM-alpha, 10% FBS, 1% Pen/Strep and 10 ng/mL PDGF). After 5 days of pre-culture, 7,000 pancreatic carcinoma cells (MIA-Paca-2) were seeded to create co-cultures and maintained for up to 2 weeks. Top view at hydrogel surface (A) and close-up inside the gel (B). Bright-field microscopy showing growth and invasion of MIA-Paca cells in association with pre-seeded fibroblasts during the first week of cancer cell culture (C-E).
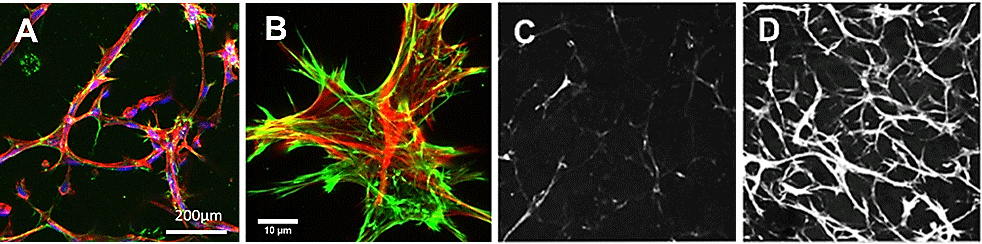
Figure 7. Extracellular matrix deposition.10,000 RFP labeled fibroblasts were seeded in the TrueGel3D® HTS Hydrogel in 200 µL of invasion medium (MEM-alpha, 10% FBS, 1% Pen/Strep and 10 ng/mL PDGF). The following day compounds to steer the ECM were added to the culture media. (A, B) Confocal micrographs of fibroblasts (red) and endogenous fibronectin (green). (C, D) Confocal micrographs of collagen deposited by fibroblast culture for 14 days without (C) or with (D) 50 g/L ascorbic acid.
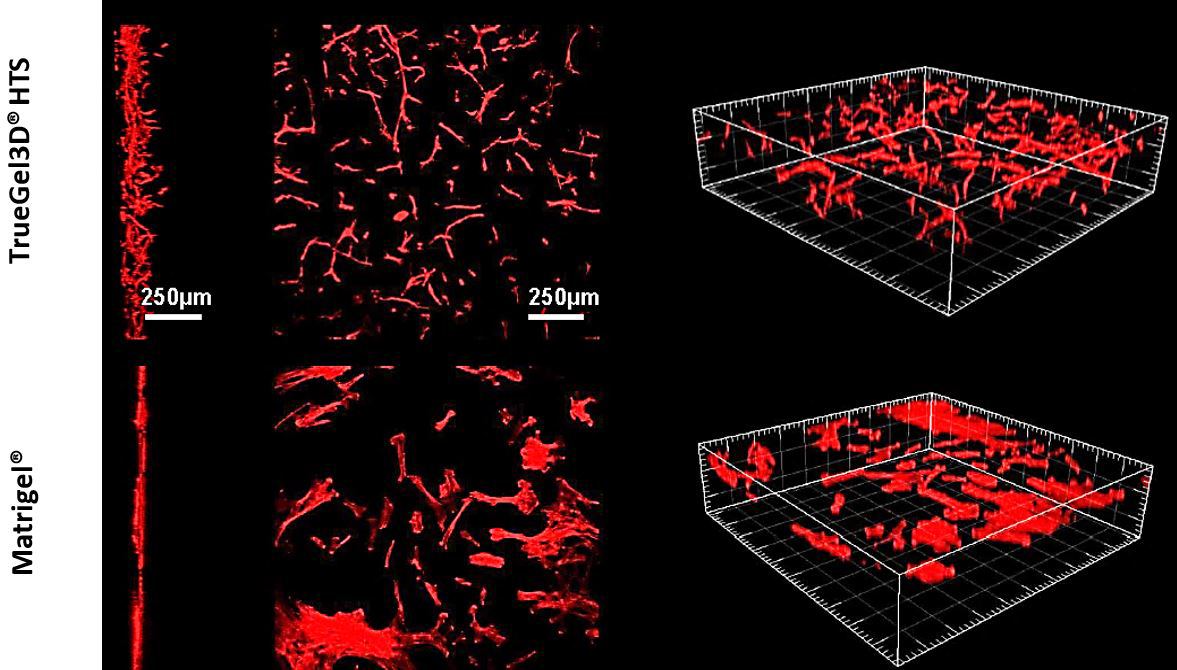
Figure 8. Human mesenchymal stem cell 3D cultures.Human adipose derived MSCs cultured in TrueGel3D® HTS Hydrogels infiltrate hydrogels and display 3D phenotypes after 7 days in culture. In contrast, hMSCs seeded on conventionally prepared hydrogels (Matrigel® matrix) grow as a monolayer on the surface of the hydrogel and do not display 3D phenotypes.
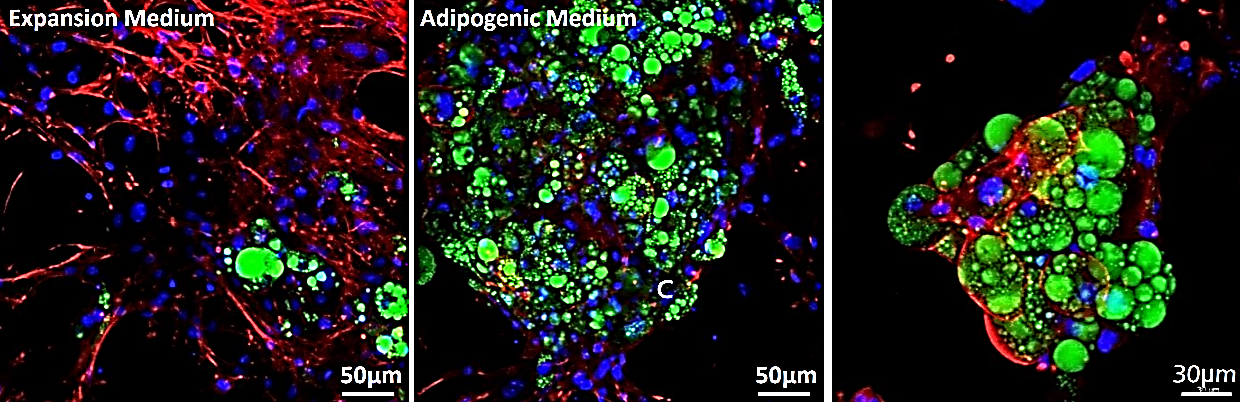
Figure 9. 3T3-L1 adipocyte differentiation. 3T3-L1 mouse fibroblasts were cultured in expansion medium (left) or adipogenesis differentiation medium (middle/right) over 21 days in TrueGel3D® HTS Hydrogels. 3D grape-like structures containing lipid vacuoles were present after 21 days of differentiation (actin (red), lipids (green) and nuclei (blue).
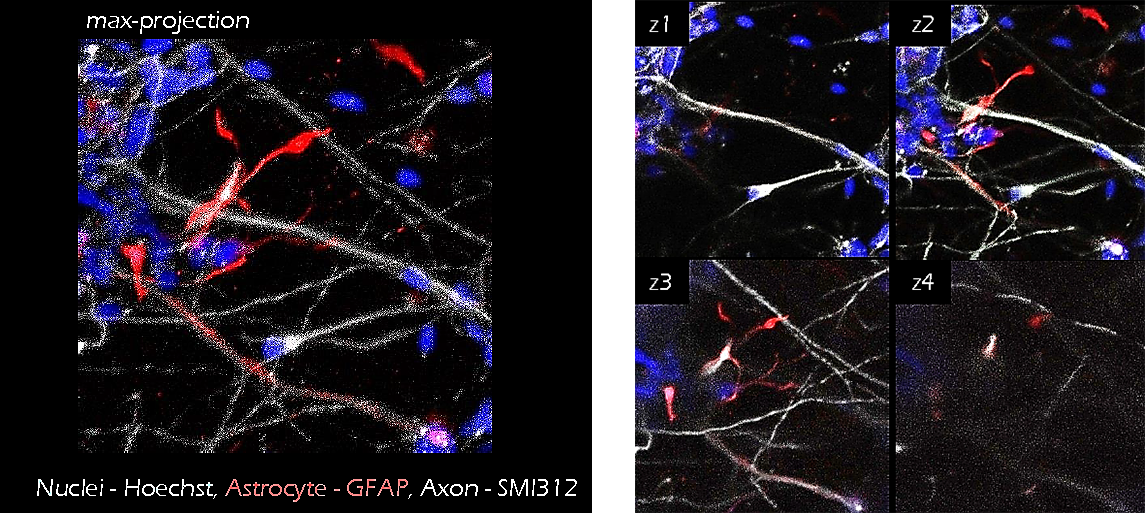
Figure 10. Neuron-Astrocyte co-culture. iPS cell derived neurons (grey) and astrocytes (GFAP, red) were co-cultured in TrueGel3D® HTS Hydrogels for 15 days. The addition of astrocytes sequentially led to higher cell viabilities and enhanced neural activity (calcium signaling, data not shown) suggesting increased cell maturation.
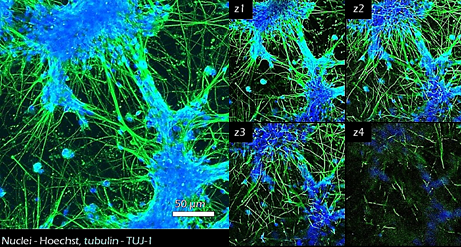
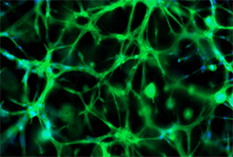
Figure 11. Neural stem cell differentiation.25,000 iPSC derived neural stem cells were cultured in TrueGel3D® HTS Hydrogels and allowed to differentiate over 7-15 days. Differentiated cells show neural phenotypes with increased TUJ-1 expression (green) and complex neural networks. Calcium imaging (GCaMP6) was also performed at day 17 (data not shown) displaying active functional calcium spiking. Subsequently, glia cells can also be added to support the maturation of the cultures and study their interaction with neurons.
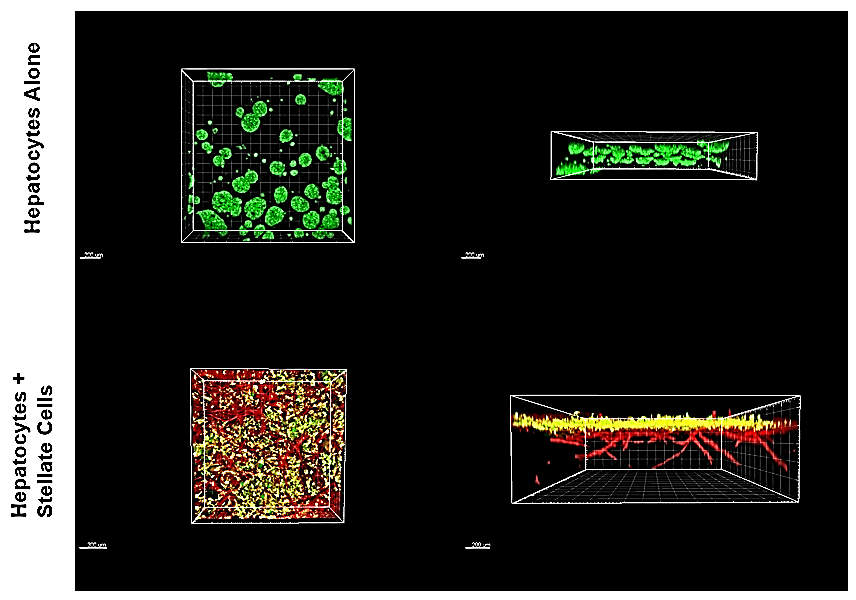
Figure 12. Hepatocytes/liver stellate cell co-cultures.HUH-7 hepatocellular carcinoma cells cultured in TrueGel3D® HTS Hydrogels tended to form spherical aggregates throughout the hydrogel. However, HUH-7 cells cultured in TrueGel3D® HTS Hydrogels pre-seeded with liver stellate cells for 5 days tended to form a monolayer on top of the stellate cells resembling liver tissue.
TrueGel3D® HTS Hydrogel Products for 3D Cell Culture
References
如要继续阅读,请登录或创建帐户。
暂无帐户?