Hydrogels for 3D Cell Culture
Introduction
What Is a Hydrogel?
Hydrogels Features and Benefits
Hydrogels Selection Guide
Introduction
Cells in their natural environment are surrounded by a complex network of extracellular molecules called Extracellular Matrix (ECM). This network provides structure and function, in the form of biochemical interactions, to the surrounding cells. Because of limitations raised by classical 2D cell culture technique where this environment is missing (or widely reduced), different approaches have been developed to tempt to mimic this extracellular environment. Among these diverse approaches (scaffold based like hydrogels or rigid scaffolds, scaffold free like low attachment plates or hanging drops for spheroids formation) the most widely used one is hydrogels.
What Is a Hydrogel?
Hydrogels can be defined as water-swollen networks of polymers. Most are liquids at 4 degrees or room temperature but will form a gel when incubated at 37 °C. Because of their properties, cells can be embedded inside hydrogels by mixing cell solution with hydrogel before gel formation: the mix is then dispensed in a cell culture vessel and during the gelation process cells will be encapsulated inside the gel. Compared to classical, 2D cell culture, cells cultured in 3D, embedded in gels, recover different characteristics they have when placed in their natural environment.
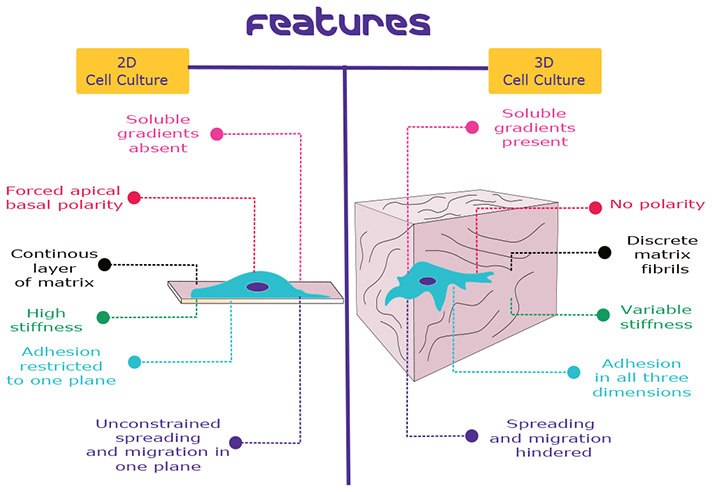
Figure 1.comparison between 2D cell culture and 3D cell culture. This picture shows the main differences between cells behaviour and constraints when cultivated in 2D environment, plated on a slide, even coated with hydrogels or any other ECM protein like collagen, compared to cells cultivated in a 3D environment, embedded in an hydrogel or any other ECM proteins.
Hydrogels Features
- Allow the cells to be placed in a more physiological shape (3 dimensions) compared to classical, flat 2-dimension cell culture
- Some hydrogels can be customized to better mimic the natural environment
- Stiffness/rigidity of the environment can be adjusted to match with natural stiffness of cell’s tissue of origin
- No need of sophisticated protocols, material or devices to grow cells in hydrogels
Hydrogel Selection Guide
The first generation of hydrogels used for 3D cell culture was use of ECM components; it was rapidly followed by more sophisticated systems, generated by doing an extraction of ECM polymers from Engelbreth-Holm-Swarm (EHS) murine sarcoma cell basal membranes (ECM based hydrogels). New generations of synthetic, hybrid or peptides-based materials have been developed since to meet specific requirements and to allow choosing the best option for each cells and applications. However, these hydrogels all show advantages and disadvantages (Table 1).
*some hydrogels are biologically active and can interact with the cells. It can sometime interfere with the results of your experiments
ECM gel (E1270, E6909): Based on the original protocols for ECM extraction from EHS murine sarcoma cell basal membranes, these hydrogel provide a very rich environment, highly compatible with cell development, but they contain mouse growth factors (E6909 is a growth factor reduced version of E1270). Major components are laminin, type IV collagen, heparan sulfate proteoglycan and entactin. These hydrogels are forming gels by thermic activation between 20 and 40 °C and the gelation process is reversible. (protein concentration: 8-12 mg / mL). ECM gel have been successfully used for several applications including analysis of contractility and invasion potential of mammary cancer cell line2, or for In vitro capillary network formation3.
MaxGel™ Human ECM: MaxGel™ human ECM is a human-based alternative to EHS murine sarcoma cell basement membrane extract. It is produced by in vitro co-culture of human fibroblasts and human epithelial cells followed by basement membrane components extraction. Like ECM gels or Cultrex gels, it contains extracellular matrix components including collagens, laminin, fibronectin, tenascin, elastin, a number of proteoglycans and glycosaminoglycans. Maxgel has been successfully used for embryoid body formation with human iPSCs4, cancer cells invasion or migration assays
Tissue Specific dECM Hydrogels: To isolate the native extracellular matrix of a tissue from its inhabiting cells, a decellularization process is employed resulting decellularized extracellular matrix (dECM) scaffolds can be used in cell culture and tissue engineering applications. Compared to other tumor-derived basement membrane extracts these dECM hydrogel scaffolds can provide a more physiological environment with increased growth rates for cells without the use of any exogenous growth factors.
PhotoGel Hydrogels: Photocrosslinked hydrogel kits contain high quality modified natural hydrogels including methacrylated collagen type-I (PhotoCol®), gelatin (PhotoGel®) or hyaluronic acid (PhotoHA®) along with photoinitiators (Irgacure® 2959, LAP, or Ruthenium) and optimized buffers and solutions to fit diverse 3D cell culture or bioprinting applications.
Hystem®: The Hystem® platform is based on chemically synthesized hyaluronic acid, one major ECM component. Depending on the cell you are studying you can chose in the platform the best suited formulation: Hyaluronic acid alone (with crosslinker), or including collagen (GelinS) with or without Heparan Sulfate. Because it is a not a biological extract, it provide a better control of the composition of cells’ environment: control over growth factor incorporation, attachment factor incorporation, ECM protein incorporation, rigidity of the hydrogel. Hystem® kits are optimal for culturing stem cells whose natural environments are rich in hyaluronic acid, but they have also been successfully used for several other applications like tissue engineering.
TrueGel3D® biomimetic synthetic hydrogels: TrueGel3D® hydrogels are biochemically defined hydrogels formed by mixing polymers with crosslinkers. They allow mimicking critical features of the natural extracellular matrix (ECM), including ECM proteins. They recapitulate native cellular environments similar to those of soft tissues, by supporting cell adhesion and protein sequestration. The TrueGel3D® hydrogels platform offer the flexibility to choose between a predefined system or to decide whether you want to customize your cellular environment (gelation time, stiffness, bioactive component integration). It has been validated for several application, including cyst formation, coculture of cancer and stroma cell, or spheroids formation.
TrueGel3D® HTS Hydrogel Plate: The TrueGel3D® HTS Hydrogel Plate is a ready-to-use solution to easily establish 3D cell cultures using fully synthetic hydrogels in a simple and automation-compatible manner. The 96-well polystyrene glass-bottom plates contain pre-casted synthetic functionalized PEG based hydrogels. These innovative hydrogels contain gradually increasing crosslinking densities throughout the well. Users can proceed directly with seeding cells without any hydrogel preparation or encapsulation steps.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?