Optimizing Upstream Media and Feeds in Bioprocessing
Developing a robust upstream cell culture process that maximizes productivity without increasing complexity relies on the right combination of bioprocess medium and feed and the ability to maintain tight control of culture conditions.
Section Overview
Cell Culture Media vs. Feed: What’s the Difference?
Your process will need both a medium and a feed to nourish your cells. In fed-batch mode, cells are initially grown in a relatively lean production medium. Over time, the nutrients become depleted and a feed is added to replenish these nutrients for the production phase. The feed can be regularly supplemented to achieve the desired product yield.
Why Optimize Your Fed-Batch Cell Culture Process?
Manufacturing titers have steadily improved over the last 40 years from tens of mg/L to 10 g/L primarily through cell line engineering, media development, and process control improvements achieving higher specific productivities (qp), increased peak viable cell densities (VCD), and prolonged production durations. Further increases in volumetric productivity have been reported, which indicates that there is still potential for additional cost reductions using fed-batch operations. Optimizing the medium and feed and media is one way to boost the productivity of your process.
As with most upstream bioprocesses, feed volumes and timings are empirically determined on a process- and cell-line specific basis to maximize performance. The reason for this is that different processes, cell-lines, and even clones vary in performance if given the same medium and feed protocol. Therefore, the medium formulations and feeding strategies must be screened, designed, and optimized for each clone.
Cell Culture Readouts to Keep in Mind While Optimizing Your Process
While optimizing your upstream process, there are several parameters to consider:
- Viable cell density (VCD): The viable cell density in combination with viability gives an indication of how many cells are in the culture as well as the state of the culture. It can inform when to begin adding additional nutrients or when to harvest the cells for downstream processes.
- Titer (volumetric productivity): Different medium and feed combinations can support different production titers. Various media and feeds can support the same VCD but could result in differences in titer.
- Glycosylation: Glycosylation affects the structure, folding ability, stability, in vivo half-life, immunogenicity, and efficacy of a drug molecule. Understanding the glycan profile is especially critical for biosimilars which must demonstrate comparable profiles to the originator molecule. The glycan profile can be determined by various parameters including the cell line, vector designs, and how clone screening and selection are conducted. Process parameters such as temperature, pH, dissolved oxygen, media, and supplements can also affect glycan profiles.
To start, begin with optimizing media and feeds that are designed to work together and adjust feed volumes and timings of feed administration examining the parameters listed above. To get the basics on optimizing and measuring these readouts when testing out feeds with your media, see our case study using Cellvento® Modifeed Prime COMP.
Key Considerations for Optimizing Media and Feeds for Upstream Cell Culture
Test Combinations of Media and Feeds
Given the variety of media and feeds available on the market, evaluating multiple options from different vendors is a common way to determine what fed-batch system represents the best option for a particular CHO cell platform. In addition to screening fed-batch systems from different vendors, media from one supplier may be evaluated in combination with a non-companion feed from another. In some cases, a multipart medium and/or feed from multiple vendors are evaluated. This approach speaks to the diversity of CHO cell lines beyond the historical lineages of CHO-S, CHO-DG44, and CHO-K1. The platforms that exist within the biopharmaceutical industry, along with the individual clones produced from those platforms, vary greatly in nutrient requirements and performance.
With little knowledge of the actual composition of medium and feed formulations on the market and with a need to use screening time effectively, all available options must be evaluated to optimize the strategy. While this approach can be effective, it may result in a situation where different vendors are supplying medium and feed, leading to increased supply chain risk.
For more detail on how to test combinations of media and feeds, see our case study optimizing two media and feed platforms.
Different Clones Perform Best Under Different Media / Feed Combinations
It’s important to optimize media and feed preferences based on the clone you are using. In evaluating three clones from an IgG cell line generated using the CHOZN® GS CHO cell line development platform with UCOE® expression technology, we show that the clones preferred different feed blends (Figure 1). For example, clone D preferred the individual Cellvento® 4Feed whereas clone E preferred the 50:50 feed blend with EX-CELL® Advanced CHO Feed 1; clone F preferred both the 50:50 feed blend and the blend with 75 percent Cellvento®4Feed (75:25).
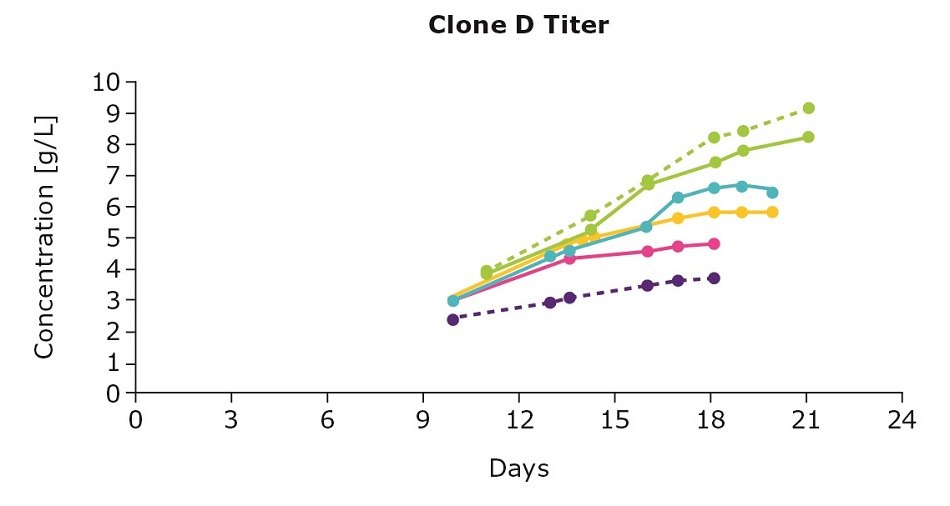
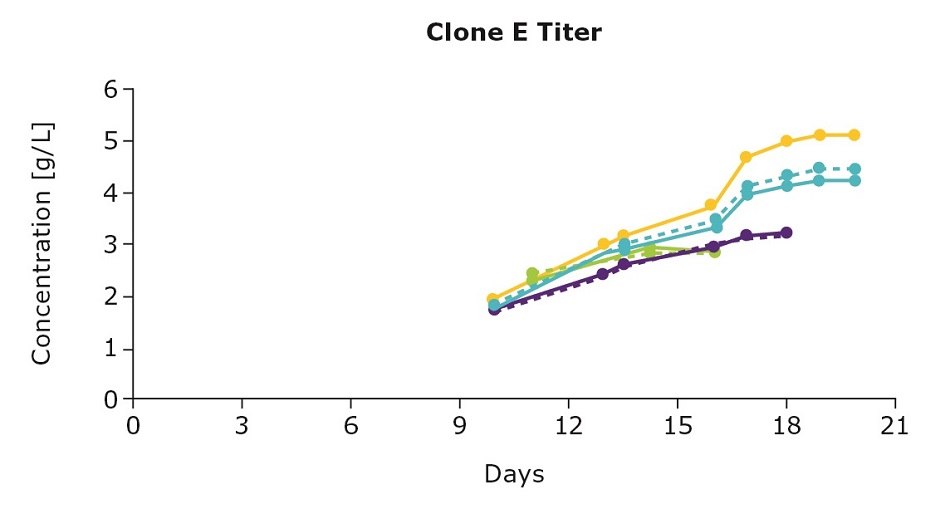
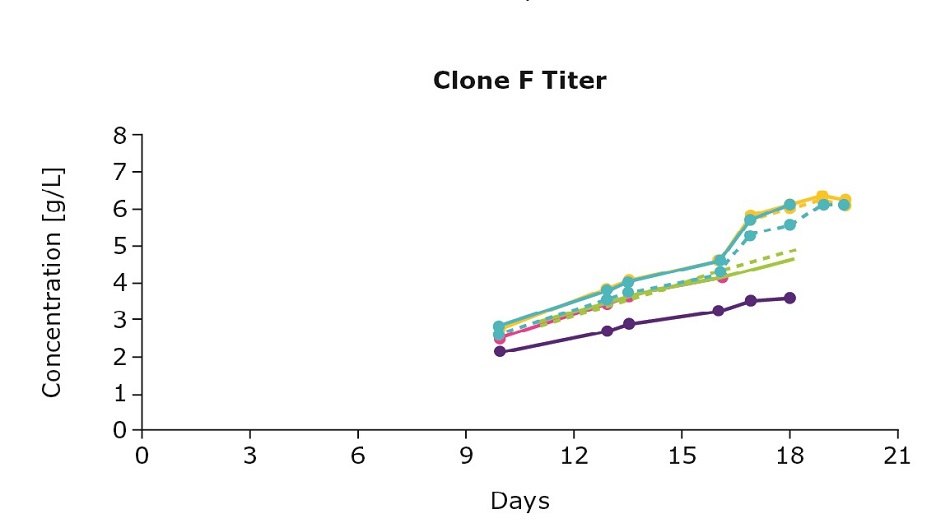
Figure 1. Feed optimization for various clones shows individual clone’s feed preferences.
Balancing Growth and Production
Although the VCD gives you an indication of the growth stage of your culture, it’s important to note that more robust growth doesn’t necessarily mean an increase in protein production. Continuing with the example above, clone D and clone E had higher viable cell density leading to higher titers. For clone F, despite the lower viable cell density, optimizing the feed improved antibody production
(Table 1).
Consider a Chemically Defined Feed
While there are many undefined feeds derived with hydrolysates, it could be useful to consider a chemically defined feed. Chemically defined feeds lower risks, have reduced lot-to-lot variation, and can facilitate desirable product attributes (ex: glycosylation). In another article, we share a case study showing the performance of the chemically defined supplement, EX-CELL® CD Hydrolysate Fusion, on mAb-producing CHO cell lines.
Minimizing the Number of Feed Lines by Using a Single Feed
To simplify your feeding process, you can also consider a single feed that contains a mixture of chemically defined components. These types of feeds, such as our EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed, reduce the number of manipulations and connections to the bioreactor especially when feeds are fed individually or blended together before introducing into the bioreactor.
While these are important considerations, this article is not an exhaustive list of the ways you can optimize your process. To find more technical information about media and feed strategies, see the Media Development application page.
Related Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?