Upstream Bioprocessing Feed Strategies and Scalability: A Case Study with Cellvento® ModiFeed Prime COMP
Prior to scaling your upstream bioprocess to bioreactors, it’s best to set up small-scale cultures to determine the media and feed combinations as well as other parameters to best optimize growth, productivity, and product quality from your cells. In this case study, we demonstrate this process using our Cellvento® ModiFeed Prime COMP feed.
Culture Growth Conditions
Two cell lines expressing mAbs, CHOZN® mAb1 and mAb2, were cultured in spin tubes containing either EX-CELL® Advanced CHO or Cellvento® 4CHO COMP fed-batch medium.
Cellvento® ModiFeed Prime COMP was fed at 17.5% total volume. Similar peak viable cell density (VCD) was observed for CHOZN® mAb1 in both media while the viability was better maintained, and productivity was highest in Cellvento® 4CHO COMP. CHOZN® mAb2 experienced higher peak VCD in EX-CELL® Advanced CHO fed-batch medium and this increase in VCD correlated with increased production. While both EX-CELL® Advanced CHO and Cellvento® 4CHO COMP fed-batch media performed well, a clonal preference was clearly demonstrated (Figure 2).
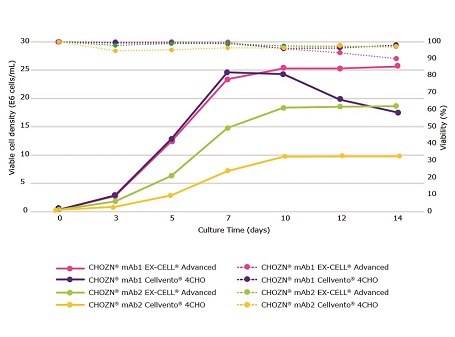
Figure 1.Cellvento® ModiFeed Prime COMP performance with different base media. Viable cell density (solid lines) and viability (dashed lines) during a 14 day fed-batch process for CHOZN® mAb1 and mAb2 cultured in EX-CELL® Advanced CHO fed-batch medium and Cellvento® 4CHO COMP.
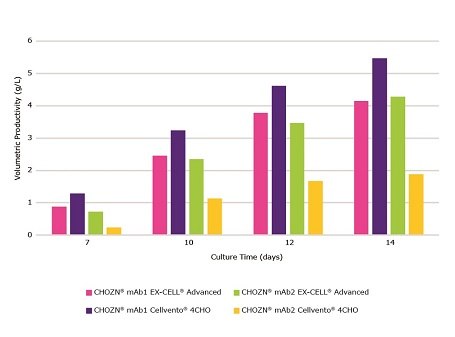
Figure 2.Cellvento® ModiFeed Prime COMP performance with different base media. Volumetric productivity during a 14 day fed-batch process for CHOZN® mAb1 and mAb2 cultured in EX-CELL® Advanced CHO fed-batch medium and Cellvento® 4CHO COMP.
Cellvento® 4CHO COMP was shown to be the best medium for use in conjunction with CHOZN® mAb1 (Figure 2). Cellvento® ModiFeed Prime COMP represents the optimum feed for use in combination with Cellvento® 4CHO COMP. Figure 3 compares Cellvento® ModiFeed Prime COMP and other commercially available feeds in spin tube cultures of CHOZN® mAb1 using Cellvento® 4CHO COMP.
Cellvento® ModiFeed Prime COMP was fed at 17.5% total feed, while other feeds were fed according to the primary recommendations in their respective product literature. While Cellvento® ModiFeed Prime COMP did not produce the highest peak viable cell density, it maintained a high viable cell density for a longer period than Competitors A and C (Figure 3). Cellvento® ModiFeed Prime COMP yielded the highest volumetric productivity on all days analyzed (Figure 4). Competitor B produced the lowest peak viable cell density and subsequently the lowest volumetric productivity.
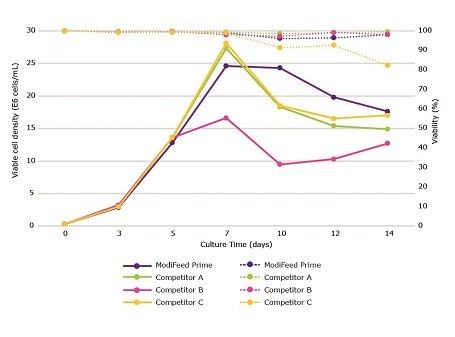
Figure 3.Cellvento® ModiFeed Prime COMP performance compared to other commercially available feeds. Viable cell density (solid lines) and viability (dashed lines) during a 14 day fed-batch process for CHOZN® mAb1 cultured in Cellvento® 4CHO COMP supplemented with either Cellvento® ModiFeed Prime COMP or other commercially available feeds.
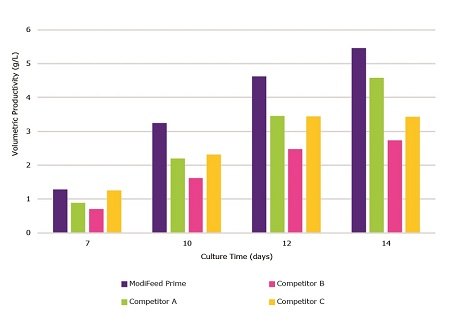
Figure 4.Cellvento® ModiFeed Prime COMP performance compared to other commercially available feeds. Volumetric productivity during a 14 day fed-batch process for CHOZN® mAb1 cultured in Cellvento® 4CHO COMP supplemented with either Cellvento® ModiFeed Prime COMP or other commercially available feeds.
Cellvento® ModiFeed Prime COMP Feeding Strategy
As with most upstream bioprocesses, optimization of feed volumes and timing of feed administration should be empirically determined on a process- and cell-line specific basis to maximize performance. Cellvento® ModiFeed Prime COMP, used in conjunction with Cellvento® 4CHO COMP or EX-CELL® Advanced CHO fed-batch medium, is recommended to be fed at between 15% and 27.5% total feed, depending on the demands of the clone(s) tested. Table 2 provides the recommended ranges for evaluation of feed percentage, feed frequency and glucose concentration.
Feeding should be initiated only when the viable cell density is ≥ 2x106 cells/ml and no earlier than day 3 to avoid over-feeding. Cultures should be terminated and harvested when viability drops below 70%.
Initial evaluations should consist of 17.5% and 22.5% total feed unless the general demands of the clone(s) are known (Table 2). Once a preference for a lower or higher total feed percentage is established, optimization through further increasing or decreasing the total feed can be evaluated. If desired, the schedule can be adjusted to support a more regular feed interval (Table 3).
The suggested initial feeding evaluation is designed to support a low seed (2-5 x105 cells/mL), 14 day fed-batch process. If viability is still high late in culture, longer fed-batch processes can be supported with additional feeding; the feeding frequency and percentage is then continued until viability drops below 80%. A high seed fed-batch (2-5 x106 cells/mL) may require adjustment in feeding schedule to support higher biomass earlier in culture and provide more total feed. Feeding can be initiated as early as day 2 to support higher biomass early in culture. High seed may represent an option for shortening the duration of longer fed-batches or for achieving increased production over the same 14 days.
Testing multiple feed strategies is important as increased feeding may result in productivity gains or may have a neutral or even negative effect on productivity for less demanding clones (Figure 5). CHOZN® mAb1 and DG44 mAb3 were cultured in spin tubes using Cellvento® 4CHO COMP and EX-CELL® Advanced CHO fed-batch media, respectively. Cellvento® ModiFeed Prime COMP was fed at 17.5% total feed (3% on days 3 and 5, 5.5% on day 7, and 3% on days 10 and 12) or 22.5% total feed (4% on days 3 and 5, 6.5% on day 7, and 4% on days 10 and 12). Cellvento® ModiFeed Prime COMP enables all high-performing CHO cell lines, CHOZN® and DG44 shown here, to achieve their full production potential.
Similar peak VCDs were observed for both clones, no matter the feeding strategy (Figure 5). CHOZN® mAb1 maintained a higher viability and VCD when fed at 22.5% total feed which led to a higher peak volumetric productivity. The amount of total feed had no impact on the VCD and viability of DG44 mAb3. An increase in total feed had a significantly higher impact on the productivity of CHOZN® mAb1 compared to DG44 mAb3 (Figure 6).
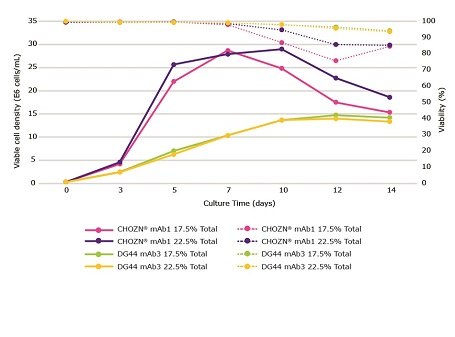
Figure 5.The impact of feeding schedules with Cellvento® ModiFeed Prime COMP using different cell lines. Viable cell density (solid lines) and viability (dashed lines) during a 14 day fed-batch process for CHOZN® mAb1 cultured in Cellvento® 4CHO COMP and DG44 mAb3 cultured in EX-CELL® Advanced CHO fed-batch medium.
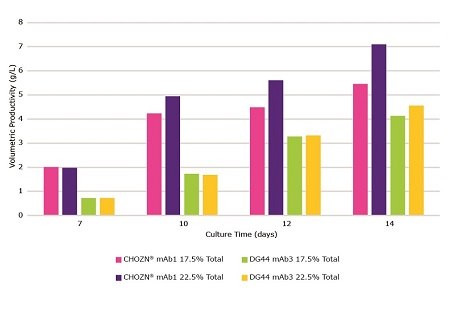
Figure 6.The impact of feeding schedules with Cellvento® ModiFeed Prime COMP using different cell lines. Volumetric productivity during a 14 day fed-batch process for CHOZN® mAb1 cultured in Cellvento® 4CHO COMP and DG44 mAb3 cultured in EX-CELL® Advanced CHO fed-batch medium.
Testing scalability from small scale to bioreactors
After optimizing your feed and media in small scale, you will next need to determine whether these conditions can scale to your bioreactor. You’ll need to ensure that the same conditions give comparable results in terms of growth, viability, productivity, glycosylation and charge variance (Figure 7).
In our case study, CHOZN® mAb1 was cultured in spin tubes, 3L Mobius® single-use bioreactors and a 50L Mobius® single-use bioreactor in EX-CELL® Advanced CHO Fed-Batch Medium (Table 4). Cellvento® ModiFeed Prime COMP was fed at 25% total feed (4.5% days 3 and 5, 7% day 7, 4.5% days 10 and 12). A high total feed percentage was defined as optimal for this clone (Figure 6) and further optimized as noted under feeding strategy (data not shown).
Cell Growth and Productivity
EX-CELL® Advanced CHO Fed-Batch Medium was used in order to confirm that an increase in productivity, over the 17.5% total feed (Figure 2), would be observed in both media when using a higher total feed percentage. CHOZN® mAb1 cultured in a 50L Mobius® single use bioreactor saw slight increases in peak viable cell density, longevity, and productivity over the 3L Mobius® single use bioreactors and spin tubes.
Glycosylation and Change Variance
Only subtle changes in the glycoprofiles (Figure 9) were observed across the three scales tested. The use of single use Mobius® bioreactors, as opposed to spin tubes, generated an increase in acidic and a decrease in main charge variants (Figure 10).
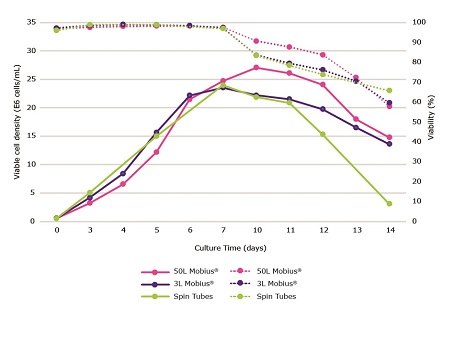
Figure 7.Comparison of viable cell density (solid lines) and viability (dashed lines) during a 14 day fed-batch process for CHOZN® mAb1 cultured in EX-CELL® Advanced CHO fed-batch medium. Cultures were grown in spin tubes, 3L Mobius® single-use bioreactors and a 50L Mobius® single-use bioreactor.
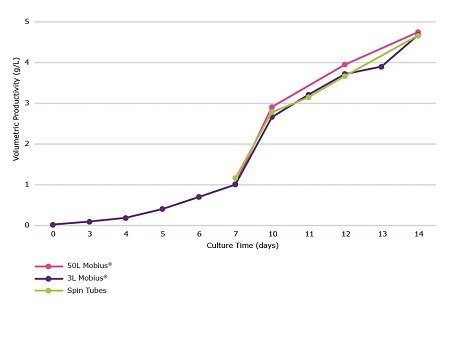
Figure 8.Comparison of volumetric productivity during a 14 day fed-batch process for CHOZN® mAb1 cultured in EX-CELL® Advanced CHO fed-batch medium. Cultures were grown in spin tubes, 3L Mobius® single-use bioreactors and a 50L Mobius® single-use bioreactor.

Figure 9.Comparison of day 14 glycoprofile during a 14 day fed-batch process for CHOZN® mAb1 cultured in EX-CELL® Advanced CHO fed-batch medium. Cultures were grown in spin tubes, 3L Mobius® single-use bioreactors and a 50L Mobius® single-use bioreactor.
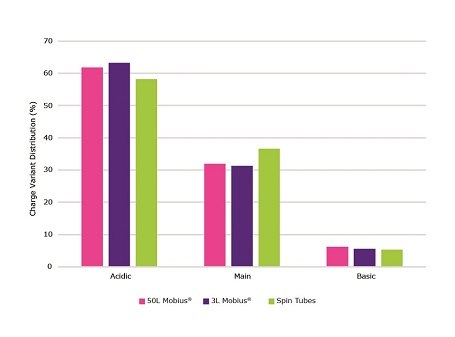
Figure 10.Comparison of day 14 charge variant distribution during a 14 day fed-batch process for CHOZN® mAb1 cultured in EX-CELL® Advanced CHO fed-batch medium. Cultures were grown in spin tubes, 3L Mobius® single-use bioreactors and a 50L Mobius® single-use bioreactor.
About Cellvento® ModiFeed Prime COMP
Cellvento® ModiFeed Prime COMP is a chemically-defined (CD), highly concentrated, single part, pH-neutral feed for replenishing depleted nutrients required for cellular function and to extend the production phase in fed-batch mode. It is formulated for fed-batch production of monoclonal antibodies (mAbs) and next-generation biopharmaceuticals in all Chinese Hamster Ovary (CHO) cell-based expression systems, in combination with Cellvento® 4CHO COMP and EX-CELL® Advanced CHO production media, or many leading base media for CHO cells.
Use of this feed reduces workflow complexity and risk to the process. The feed is supplied as a compacted dry powder which reduces the bulk volume, improves flowability, decreases dust formation, simplifies handling and accelerates dissolution. Once hydrated, the feed can be stored protected from light at 2-8°C for up to 90 days or at room temperature for up to 30 days.
The advantages of Cellvento® ModiFeed Prime COMP are summarized in Table 5.
Products
To continue reading please sign in or create an account.
Don't Have An Account?