Media Development
SELECTING THE RIGHT MEDIA AND FEED STRATEGY
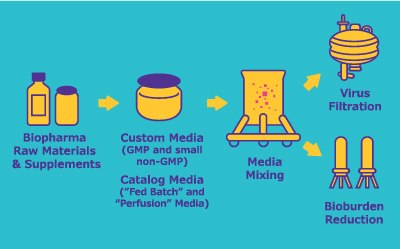
To maximize performance of cell lines, biopharma manufacturers must find the right media, relevant to their production mode. For instance, perfused seed trains and production bioreactors require new media formulations for optimal performance.
Feed strategies must be based on empirical data from media screening and optimization, as well as nutrient testing. Feed volumes and timing of feed administration should be determined for each specific process and cell line via high throughput (HTP) and design of experiment (DOE) approaches. These can be conducted internally or by partnering with experts to expedite simultaneous screenings.
Featured Categories
Explore Mobius® single-use mixing solutions for pharmaceutical ingredient blending and process solution preparation.
Our off-the-shelf and customizable bioprocessing cell culture media (CCM) products enhance productivity in upstream mAb, vaccine, gene/cell therapy processes.
The Mobius® Bioreactor family offers single-use bioreactors for cell culturing from early development to commercial production, supporting various scales.
Explore our solutions for process solution buffer and media preparation, including our Mobius® single-use mixing and Mobius® FlexReady solutions.
SAFE HANDLING AND EFFICIENT MIXING OF DRY POWDER CELL CULTURE MEDIA
Dry powder cell culture media formulations provide many advantages for shipping and storage. To leverage these benefits, dry powder media formulations need to be highly soluble and homogeneous. These properties can be achieved by the combination of optimized milling procedures and suitable formulation technologies. Additionally, media should arrive in a format that is convenient to handle, such as pre-weighed dry powder bags that connect directly to hydration tanks. This minimizes product contamination risks, safeguarding employees and facilities. When mixing, especially at high volume, good dispersion and dissolution of particles can be managed with specially designed single-use mixing systems.
Intensifying the Seed Train using HCDC and Specially Designed Expansion Media
From thawing a single vial of cells, to expanding cells for a GMP manufacturing batch run is time-consuming, labor intensive, and requires open cell culture operations. Use of a High Cell Density Cryopreservation (HCDC) method, which feeds into the first seed train bioreactor, can streamline the overall process. As the frozen volume is relatively high, single-use bags are more appropriate than vials. This allows HCDC to be used in closed processing, thus reducing contamination risks.
Perfusing the seed train is a solution for achieving higher cell densities, and it is recommended to use specially designed expansion media for an optimal result. It enables high seed inoculation of your production bioreactor through higher cell biomass or reduces your upstream footprint by decreasing the number of bioreactors in the seed train. In addition, it saves time by eliminating adaptation during transfers from the vial thaw to expansion and production.
REDUCING VIRAL CONTAMINATON RISKS WITH NON-ANIMAL ORIGIN RAW AND CHEMICALLY DEFINED RAW MATERIALS
Contamination often originates from raw materials and animal-derived components such as bovine serum or trypsin. Wherever possible, raw materials and animal-derived components at high risk of virus contamination should be replaced with low(er)-risk alternatives, such as chemically defined cell culture media and non-animal origin recombinant supplements. They also simplify regulatory processes.
OVERCOMING INSOLUBLE OR UNSTABLE AMINO ACIDS
The essential amino acids tyrosine and cysteine present a challenge when used in upstream processing, due to low solubility (tyrosine) and low stability (cysteine) at neutral pH. To simplify fed-batch processes and enable high available concentrations of both amino acids, chemically modified tyrosine and cysteine enable a single feed strategy at neutral pH. Other benefits of this approach include reduction of feed volume and increased volumetric productivity.
ENSURING LOT-TO-LOT CONSISTENCY OF RAW MATERIALS
Lack of consistency in raw materials can create significant variation in upstream processes, causing unexpected loss of cell density and viability – and ultimately, reduced yield. A better understanding of raw materials increases ability to control process variabilities. This can be achieved by tracing the supply chain back to raw materials and characterizing them.
EXPEDITING RAW MATERIAL QUALIFICATION
Current regulatory and industry guidelines do not define clear standards for chemicals used in upstream bioprocessing. In the absence of standards, drug manufacturers rely heavily on their suppliers for efficient sourcing and qualification of raw materials; however, typical timelines to collect raw material information via questionnaires and separate statements can be unacceptably long, often taking several weeks. Consequently, manufacturers are seeking supply partners who can provide raw materials that are stringently qualified to industry-leading standards and are supported by comprehensive documentation packages that meet pharmaceutical manufacturers’ information needs when qualifying raw materials, completing a risk assessment or optimizing a manufacturing process.
Visit our document search for data sheets, certificates and technical documentation.
Find More Articles and Protocols
Related Product Resources
- Tech Brief: Lay the Proper Foundation for Upstream Success
You need speed, quality and regulatory compliance for development of your upstream processes.
- White Paper: Turnkey Solutions to Improve Cell Culture Performance
This whitepaper describes two turnkey upstream solutions that can be applied to improve the performance of monoclonal antibody (mAb) production clones and set the stage for a successful manufacturing process.
- Application Note: Improving Fed-batch Yields by Combining EX-CELL® Advanced and Cellvento® Cell Culture Media Portfolios
This application note provides guidance and options for improving culture yield with off-the-shelf catalog products.
- Flyer: Media Selection Guide
Our brands brands offer a comprehensive range of off-the-shelf products to support all of your CHO production processes.
- White Paper: Optimizing Media Feed Strategies for Cellvento® CHO Media Platform
- Flyer: Cellvento® and EX-CELL Feed Mixing Protocol
- Application note: Process guidance Cellvento® 4CHO Fed-batch Medium
- White paper: Strategies for Upstream Intensification
- White Paper: A Cost Analysis and Evaluation of Perfused Seed Train Scenarios Through Process Modeling
- White Paper: Seed Train Intensification Using High Cell Density Cryopreservation and Specially-designed Expansion Medium
- White Paper: Upstream Intensification – Enabling Perfusion Processes with Cell Retention Technologies
- White Paper: Guidelines for Developing a High Cell Density Cryopreservation Process
- Application Note: Preparing CHO Cells for Higher Productivity by Optimizing a Perfused Seed Train
- White Paper: Mitigating Risks Associated with Cell Culture Media Preparation and Handling
- Article: Innovative Chemicals for Process Intensification in Cell Culture Media
- White Paper: Identifying Appropriate-quality Raw Materials in an Evolving Regulatory Environment
- eBook - Cell Culture Media – Supply Robustness and Control
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
Workflow

Cell Line Development
Development begins by selecting single-cell clones that can produce the biologic of interest, then screening for clones that are stable, productive, and scalable
Bioreactor Production
Choosing an upstream platform must take many considerations into account, including scalability and quality control of the bioreactors and mixers

Monoclonal Antibody Manufacturing
Monoclonal antibody manufacturing is a highly templated approach used to produce mAb-based immunotherapies. Robust, scalable process solutions are required at every step to ensure high therapeutic concentration and process safety, while meeting speed-to-market and cost containment concerns.
To continue reading please sign in or create an account.
Don't Have An Account?