Improving Fed-batch Yields in Upstream Bioprocessing
The biopharmaceutical industry primarily uses fed-batch methods, rather than continuous or perfusion approaches, to produce stable monoclonal antibodies (mAbs) and next generation biopharmaceuticals from Chinese hamster ovary (CHO) cells. Because different CHO platforms and clones vary in their nutrient requirements, medium and feed formulations and feeding strategies must be screened, designed and optimized for each clone. In this article, we’ll walk through media and feed screening strategies suitable for many cell lines and manufacturing scenarios using our EX-CELL® Advanced and Cellvento® Fed-batch platforms.
Section Overview
How to Evaluation of Media and Feeds in Combination
Screening multiple medium and feed pairs offers one of the best ways to arrive at an optimal fed-batch combination for a given cell line. Combining various media and feed platforms offers even more opportunities to arrive at the optimal combination. These tests should examine whether specific combinations improve cell growth, culture longevity, productivity, and product quality.
In a nutshell, the following are the steps to take to determine the best media and feed combinations and methodology.
1. Test a Minimal Set of Media and Feed Combinations
To start off, screen a relatively small number of combinations of media and feeds. In our initial case study described below, we tested out two different media with two different feeds as shown in Table 1.
The 50:50 mixture of feeds listed in Table 1 can be delivered by combining the individual liquid feeds into a single mixture prior to supplementing or by feeding each individually. By creating a single liquid mixture, a single feed operation is possible. Table 2 provides an example of a representative 50:50 feeding strategy for the feeds when used independently.
2. Further Optimize Feed Ratios to Maximize Performance
Given the differences in feed characteristics, along with the variability in nutrient requirements and behaviors of every cell line, additional ratios of feeds should be examined if possible. Additional ratios can include 75:25 and 25:75 along with 67:33 and 33:67 if desired. The single mixture of these feed ratios can be stored, protected from light, at 2-8°C for up to 30 days.
While typical feed volumes and frequency ranges are provided in Table 3, it is recommended that the needs of each cell line be empirically determined.
3. Further Evaluate Optimal Volumes and Timing
Following an initial evaluation of the medium and feed(s), a follow up study should be conducted to determine optimal volumes and timing for a given cell line.
Case Studies: Evaluation of mAb Expressing CHOK1 and CHOZN® Cell Lines Cultured using EX-CELL® Advanced and Cellvento® Media and Feeds
The following case studies evaluated whether synergies could be achieved through combining the EX-CELL® Advanced and Cellvento® Fed-batch Systems using CHOK1 and CHOZN® cell lines expressing proprietary mAbs.
Initial studies were conducted in 50 mL spin tubes, as this system offers high throughput and is scalable to bioreactors. This approach also permits near simultaneous analysis of multiple experimental conditions. Shake flasks, deep well plates, and micro-bioreactors are other suitable platforms with which to conduct evaluation studies. The final study was conducted in a Mobius® 50L Single-Use Bioreactor.
For small scale experiments, cell count, viability, and volumetric productivity were determined on specified days using a ViCell™ XR counter and ForetBio Octet® QKe system. Productivity was determined using a standard curve generated from a representative mAb. Individual conditions were maintained until day 14 (no condition had a cell viability drop below 70%), at which time the experiment was terminated. All small-scale data points shown are the average of biological duplicates. For the bioreactor experiment, volumetric productivity was determined using a HPLC based methodology and single data points are shown.
It is important that the media used during cell expansion and production are the same or known to be compatible. In this case, cell lines were adapted to each medium for at least 3 passages, cells were expanded and the fed-batch performed in the same medium. Refer to the product information sheets for the EX-CELL® Advanced CHO Fed-batch Platform and Process Guidance for Cellvento® 4CHO Fed-batch Platform.
Materials
- CHOK1 cell host expressing proprietary mAb (CHOK1 mAb1) and CHOZN® ZFN-modified GS-/- CHO Cell Line expressing a different proprietary mAb (CHOZN® mAb2). The CHOZN® mAb2 cell line utilizes a plasmid containing UCOE® elements alongside mAb2, helping to achieve high volumetric productivity.
- EX-CELL® Advanced CHO Fed-Batch Medium pH 7.2
- EX-CELL® Advanced CHO Feed 1 pH 8.5 (without glucose)
- Cellvento® 4CHO pH 7.0
- Cellvento® 4Feed pH 7.0
- Glucose stock solution (400 or 450 g/L)
- Climo-Shaker ISF1-XC - 320 rpm, 25 mm orbital, 37°C, 5% CO2, 80% relative humidity
- TubeSpin® Bioreactor 50
- ViCell™ XR counter - viable cell density and viability determination
- ForetBio Octet® QKe system - mAb quantitation of small scale experiments
- Mobius® 50L Single-Use Bioreactor
Study 1: Initial Screening Identifies Combination of Media and Feeds that Result in Higher Protein Production
In the first study, the CHOK1 mAb1 cell line expressing a proprietary mAb was grown in 50 mL spin tubes with multiple medium and feed combinations as described in Table 4. The study design paired EX-CELL® Advanced and Cellvento® products, evaluated the possible usefulness of a 50:50 combined feed and maintained the individual fed-batch controls. The feeding schedule utilized for the 50:50 mixture was dictated by the medium used and aligned with the schedule used by each system’s feed.
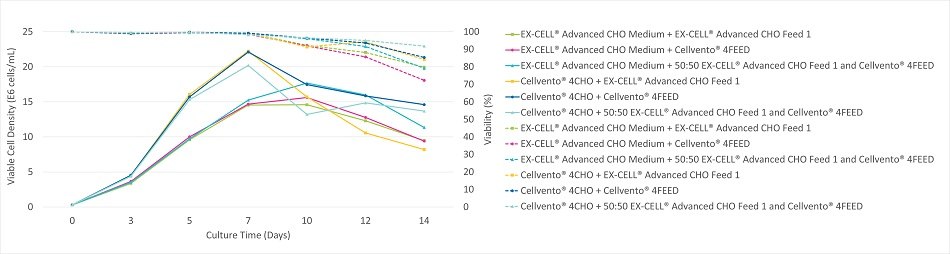
Figure 1A.Viable cell density (solid lines), viability (dashed lines) during the 14 Day fed-batch process for CHOK1 mAb1 cultured in Cellvento® 4CHO and EX-CELL® Advanced CHO Medium with each single feed and mixed (50:50) feed.

Figure 1B.Volumetric productivity during the 14 Day fed-batch process for CHOK1 mAb1 cultured in Cellvento® 4CHO and EX-CELL® Advanced CHO Medium with each single feed and mixed (50:50) feed.
The growth curves (Figure 1A) were clustered into two groups depending on the medium used. Conditions containing EX-CELL® Advanced CHO Fed-Batch Medium grew more slowly and had peak viable cell densities between 14.5 and 17.5x106 cells/mL on day 10. Conditions containing Cellvento® 4CHO medium grew more quickly and had peak viable cell densities between 20 and 22x106 cells/mL on day 7. The impact of the feed on growth was dependent upon the medium with no universal pattern for this clone. The viability curves (Figure 1A) express similar cell line preferences as those seen for viable cell density. Prolonged viability was generally observed for CHOK1 mAb1 in the conditions containing Cellvento® 4CHO medium. The volumetric productivity (Figure 1B) varied significantly with each experimental condition.
For CHOK1 mAb1, Cellvento® 4CHO medium yielded the highest productivity when fed 50:50 Cellvento® 4Feed and EX-CELL® Advanced CHO Feed 1 while EX-CELL® Advanced CHO Fed-Batch Medium yielded the highest productivity when fed Cellvento® 4Feed. These top producing conditions did not correspond with the highest peak viable cell densities (Figure 1A) suggesting that the feeds provided some nutrients required for production but not for growth. After 14 days, the highest producing combination, Cellvento® 4CHO medium fed 50:50 Cellvento® 4Feed and EX-CELL® Advanced CHO Feed 1, yielded over 45% more product than the lowest producing combination, EX-CELL® Advanced CHO Fed-Batch Medium fed EX-CELL® Advanced CHO Feed 1. This combination resulted in higher volumetric productivities than the individual fed-batch systems used as directed in their respective product information/process guidance documents. This study validated this approach as a method for potentially obtaining superior performance through a unique combination of medium and feed.
Study 2: Varying the Ratios of Feeds Further Improved Productivity
The second study used the conditions described in Table 5. Both the CHOK1 mAb1 cell line used in the first study and the higher producing CHOZN® mAb2 cell line producing a different proprietary mAb were grown in 50 mL spin tubes. The study design builds upon the work of Study 1 by evaluating additional feed ratios to determine if there was a universally optimum ratio of EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed or if the optimum ratio depends upon the cell line used. The impact of media could also be further evaluated through the continued use of both EX-CELL® Advanced CHO Fed-Batch Medium and Cellvento® 4CHO.
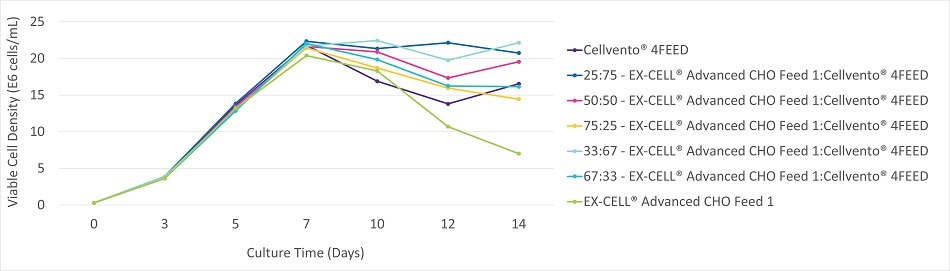
Figure 2A.Viable cell density during the 14 Day fed-batch process for CHOK1 mAb1 cultured in Cellvento® 4CHO.

Figure 2B.Viability during the 14 Day fed-batch process for CHOK1 mAb1 cultured in Cellvento® 4CHO.
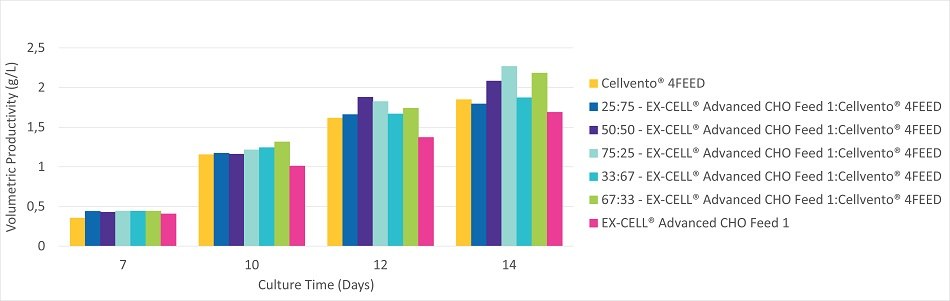
Figure 2C.Volumetric productivity during the 14 Day fed-batch process for CHOK1 mAb1 cultured in Cellvento® 4CHO.
CHOK1 mAb1 cultured in Cellvento® 4CHO showed a preference for feed mixtures containing higher amounts of EX-CELL® Advanced CHO Feed 1. Cultures fed mixtures high in EX-CELL® Advanced CHO Feed 1 experienced slightly better longevity and increased productivity as compared to mixtures high in Cellvento® 4Feed. EX-CELL® Advanced CHO Feed 1 alone experienced an earlier drop in viability (Figure 2B) and, as such, productivity was less than mixtures containing at least some Cellvento® 4Feed. Peak viable cell density was similar for all mixtures (Figure 2A). While mixtures high in Cellvento® 4Feed maintained high viable cell density for a longer time, that higher VCD did not translate into more production (Figure 2C). CHOK1 mAb1 cultured in EX-CELL® Advanced CHO Fed-Batch Medium showed similar preferences for mixtures containing high amounts of EX-CELL® Advanced CHO Feed 1 (data not shown).
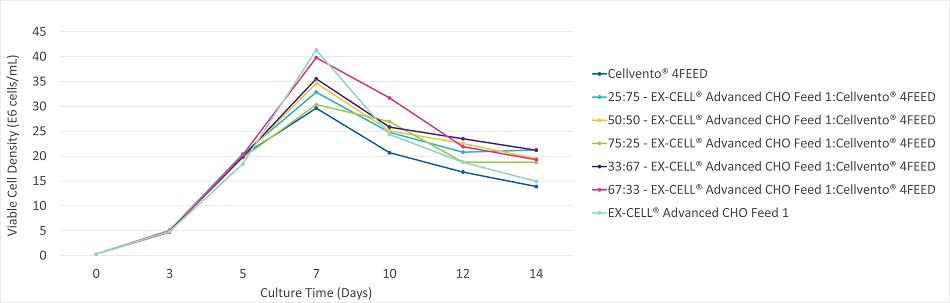
Figure 3A.Viable cell density during the 14 day fed-batch process for CHOZN® mAb2 cultured in EX-CELL® Advanced CHO fed-batch medium.

Figure 3B.Viable cell density during the 14 day fed-batch process for CHOZN® mAb2 cultured in EX-CELL® Advanced CHO fed-batch medium.
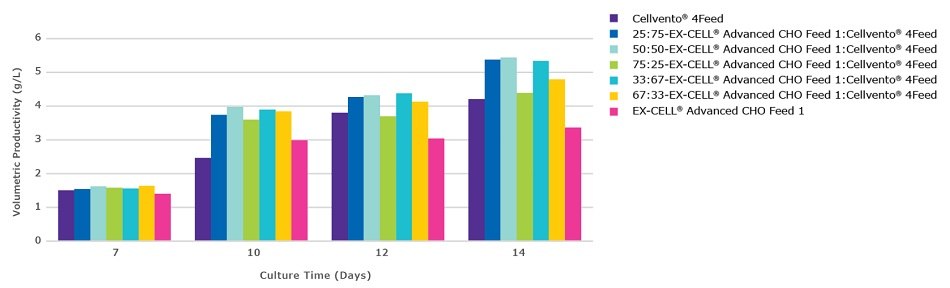
Figure 3C.Viable cell density during the 14 day fed-batch process for CHOZN® mAb2 cultured in EX-CELL® Advanced CHO fed-batch medium.
CHOZN® mAb2 cultured in EX-CELL® Advanced CHO Fed-Batch Medium showed a preference for mixtures containing higher amounts of Cellvento® 4Feed. All mixtures containing at least 50% Cellvento® 4Feed showed equivalent peak volumetric productivity (Figure 3C). Peak viable cell density varied by up to 9.5x106 cells/mL between mixtures (Figure 3A). In contrast to Cellvento® 4Feed and EX-CELL® Advanced CHO Feed 1 alone, all mixtures maintained high viability throughout the fed-batch run (Figure 3B). Cellvento® 4Feed, an important part of the high performing mixtures, experienced the lowest peak viable cell density when used alone and as such one of the lowest peak volumetric productivities. CHOZN® mAb2 cultured in Cellvento® 4CHO showed similar preferences with mixtures containing at least 67% Cellvento® 4Feed showing equivalent peak volumetric productivity (data not shown).
The results of this case study revealed a clonal preference in terms of the mixture of EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed. The impact of evaluating at least 75:25, 50:50 and 25:75 mixtures was observed, with CHOK1 mAb1 demonstrating the highest productivity when a 75:25 EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed mixture was used and CHOZN® mAb2 when a 50:50 or 25:75 mixture was used. Regardless of whether EX-CELL® Advanced CHO Fed-Batch Medium or Cellvento® 4CHO was used, the CHOK1 mAb1 cell line preferred mixtures containing higher levels of EX-CELL® Advanced CHO Feed 1 while CHOZN® mAb2 preferred mixtures containing higher levels of Cellvento® 4Feed. In both cases, the preferred mixtures maintained viabilities above 85% throughout the 14 day process with peak viable cell densities on day 7 of between 20 and 35x106 cells/mL.
Study 3: Evaluating the scalability of a 50:50 mixture of EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed
In the third study, the high producing CHOZN® mAb2 cell line was grown using EX-CELL® Advanced CHO Fed-Batch Medium in a Mobius® 50L single-use bioreactor (Table 6). Scalability of a 50:50 mixture of EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed was confirmed using one of the highest producing fed-batch conditions for CHOZN® mAb2 determined in study 2.
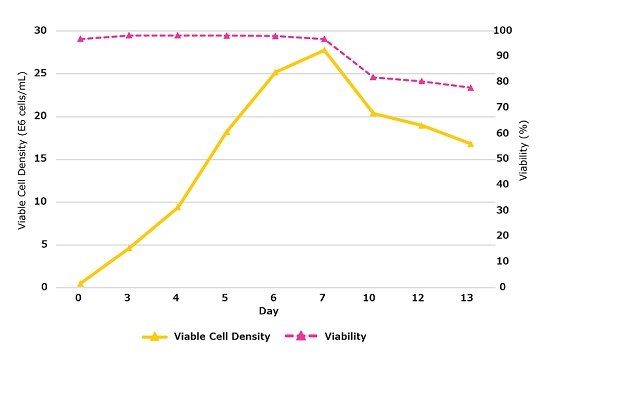
Figure 4A.Viable cell density (solid line), viability (dashed line) during the 13 Day fed-batch process for CHOZN® mAb2 cultured in a Mobius® 50L single-use bioreactor using EX-CELL® Advanced CHO fed batch medium fed 50:50 EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed.

Figure 4B.Viable cell density (solid line), viability (dashed line) during the 13 Day fed-batch process for CHOZN® mAb2 cultured in a Mobius® 50L single-use bioreactor using EX-CELL® Advanced CHO fed batch medium fed 50:50 EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed.
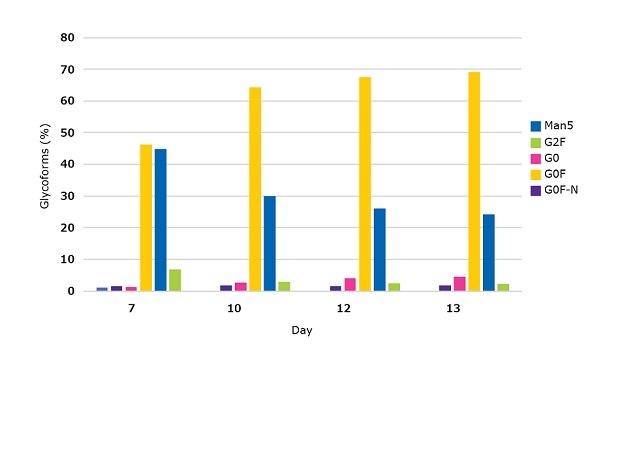
Figure 5.N-glycosylation profile during the 13 Day fed-batch process for CHOZN® mAb2 cultured in a Mobius® 50L single-use bioreactor using EX-CELL® Advanced CHO fed-batch medium fed 50:50 EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed.
When cultured in a Mobius® 50L single-use bioreactor, CHOZN® mAb2 reached a peak viable cell density on day 7 of more than 27x106 cells/mL with a viability over 96% (Figure 4A). Volumetric productivity reached more than 4.5 g/L on day 13 (Figure 4B). Comparable performance for the 50:50 EX-CELL® Advanced CHO Feed 1 and Cellvento® 4Feed mixture was achieved in with spin tubes (Figure 3C) and a Mobius® 50L Single-use bioreactor (Figure 4B). In the bioreactor, only subtle changes in the glycoprofile were observed following day 7. On day 13, this process yielded a high percentage of G0F, a significant percentage of G1F, less than 5% of G0F-N, G0, and G2F and no Man5 (Figure 5).
Conclusion
While no single fed-batch system is applicable for all clones and situations, these case studies validate the approach of combining media and feed platforms to identify the optimum combinations. Mixing and matching products provides the opportunity to explore a more diverse range of cellular preferences in an efficient manner. Only through screening of various feed ratios, bolus volumes and timings can the optimal performance be achieved. It is possible that, even in these cases, additional performance could have been achieved through further manipulation of the feeding volume and timing alone or in combination with further variation of feed ratios. Complete optimization would require additional experimentation to fine-tune the fed-batch performance.
Performance is not always directly linked to total nutrients delivered. A subset of nutrients may be critical to a cell line and subsequently lead to seemingly unexpected improvements in performance. As every cell line responds differently, an approach that evaluates feeds alone and in combination offers the best opportunity to maximize performance through delivery of the optimal mix of highly concentrated and diverse media and feed. A comprehensive evaluation of media and feeds can result in increased cell densities which support improved volumetric productivity and deliver important cost efficiencies.
About the Platforms
The EX-CELL® Advanced and Cellvento® Fed-batch platforms are animal component free, chemically defined cell culture media and companion feeds designed for CHO cell-based bioproduction.
Because no single fed-batch system will perform optimally for all CHO cells, these systems were independently developed for use with CHOZN® GS-/-, CHO-S, CHO-DG44, and CHO-K1 cell expression systems. Owing to the independent development of these two systems, they offer distinct approaches to the fed-batch process and are uniquely positioned for use in combination.
The EX-CELL® Advanced Fed-batch platform was designed based on years of experience with multiple CHO cell lines from different sources to yield superior growth, production, and longevity by replenishing depleted nutrients. This platform delivers a diverse set of nutrients to satisfy cellular requirements and is an integral part of the CHOZN® Expression Platform.
The CHOZN® platform can also be integrated with the UCOE® expression technology to accelerate biopharmaceutical development by increasing the efficiency of isolating high producing clones.
The Cellvento® Fed-batch platform was designed to provide a highly concentrated one-part feed meant to reduce total feeding volume. This single part system takes the place of a two part feed system which included a main feed and an additive feed of critical amino acids that required high pH for dissolution. The critical amino acid components are now incorporated into the main feed with modifications enabling them to be added at neutral pH to the culture. With Cellvento® 4Feed, component solubility and stability challenges were overcome and a neutral final pH was achieved.
Cellvento® 4CHO and EX-CELL® Advanced Medium are recommended for use with all CHO cell lines. Additional guidance on use of these products along with other current generation catalog formulations can be found in “CHO Cell Culture Media & Feeds Selecting the Right Media to Enable Your Next Breakthrough.”
To continue reading please sign in or create an account.
Don't Have An Account?