Neural Stem Cell Culture Protocols
Introduction
Neural stem cells are characterized by their ability to 1) self-renew and to 2) generate the different cell types found in the central nervous system including both neural and glial subtypes. Isolation and in vitro analysis of neural progenitor cell populations have been important for deciphering the cellular and molecular mechanisms underlying neurogenesis, and for optimizing stem cell-based treatment of neurological disorders and injuries. In the adult mammalian brain, NSCs exist mainly in two neurogenic regions: the subgranular zone of the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles. Recently, the use of pluripotent stem cells to make patient derived neural progenitors have aided to generate more relevant “disease-in-a-dish” cellular models of many age related neurological diseases.
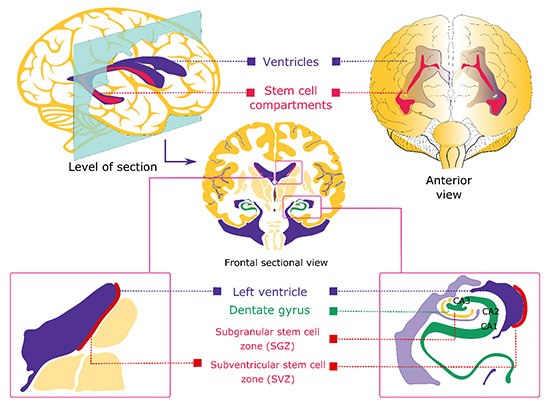
Figure 1. Regions of neurogenesis in the mammalian brain. Proliferating neural stem cells are found in the subgranular zone (SGZ) of the hippocampal dentate gyrus and subventricular zones (SVZ) of the adult and developing mammalian brain.
Methods
Isolation of Neural Stem Cells
Isolation of NSCs from Neural Tissue
- Mice or rats were anesthetized by an intraperitoneal injection of either 230 mg/kg Sodium Pentobarbital or 400 mg/kg Avertin, followed by euthanasia.
- Remove the brain and transfer to a 50-mL tube to a 10-cm petri dish (CLS430591) containing 20 mL of cold Solution A (1XHBSS (H8264) containing 30 mM glucose (G7021), 2 mM Hepes (H0887), 26 mM NaHCO3 (S5761)). Place a small piece of filter paper onto Tissue Chopper, slightly wet the filter paper using a wet sterile swab, and then set the brain onto the wet filter paper using curved-pointed forceps. Chop brain into 400-uM coronal sections and use wet sterile swab to collect the sections containing SVZ (~6 sections) and hippocampus (~5 sections) into a 6-cm petri dish (CLS430589) filled with 5 mL of Solution A.
Critical step: Keep the petri dish containing brain sections and solution A on ice during the chopping and dissection. - Dissect out the SVZ and the Dentate Gyrus (Brain Map) under a dissecting microscope and keep the dissected tissue in separate 15-mL Falcon tubes (CLS430791), each containing 10 mL of cold Solution A.
Critical step: This and subsequent steps are optimized for isolating NSCs from the SVZ and DG preparation of one mouse. If you plan to isolate cells from pooled tissues of more than one mouse, we recommend that you add the step of Percoll (P4937) purification to remove excess myelin and other cell types - After finishing the dissection of all the brain sections, spin down the tissue chunks in a low-speed centrifuge at 200 g for 1 min at room temperature (20–25 °C).
- In the TC hood remove the supernatant and add 1 mL of 0.05% Trypsin-EDTA (59417C) to each 15-ml conical tubes containing tissue chunks. Rotate the conical tubes at room temperature for 10-20 min.
Caution: Do not incubate longer than 30 min, as this will decrease the viability of the cells. - Add 1mL µL of Trypsin Inhibitor Solution (T7659) into each tube and rotate for another 10-20 min.
Caution: Do not incubate longer than 30 min, as this will reduce the viability of the cells. - Pre-wet a fire-polished glass pipette to triturate tissue by pipetting up and down 10–20 times until there is no tissue clump, and then add 8 mL of N2 medium (DMEM/F-12 (D6429), N2 supplement (SCM012), L-glutamine (G7513) to each tube. Pellet the cells at 200 g for 5 min at room temperature.
Caution: Avoid generating air bubbles when triturating the tissue, as this will reduce the viability of the cells.
Critical step: It is important to dissociate the SVZ and the DG to single cells, as any remaining aggregates can result in reduced yield. - Wash cells twice with 10 mL of N2 Medium and spin down at 200 g for 5 min at room temperature each time.
- If you are isolating cells from pooled tissues of 2 or more mice, re-suspend each cell pellet with 5.5 mL of N2 medium, add 5.5 mL of Percoll/PBS solution, and mix by inverting the tubes. Pellet the cells at 400 g for 15 min at room temperature. Then wash the cells 3 times with 10 mL N2 medium and spin down at 200 g for 5 min at room temperature each time to collect cells.
Critical step: Gently remove the supernatant, as the pellet may not be firmly attached to the bottom of the tube. Critical step: It is critical to remove Percoll by washing thoroughly, as residual Percoll will affect cell viability. - Wash one more time with 8 mL of Initial Proliferation Medium (IPM) (Neural Stem Cell Basal Medium (SCM003), B27 (SCM013), L-glutamine (G7513), 1X Pen-Strep (P4333), 20 ng/mL of FGF-2 (F0291), and 20 ng/mL EGF (E9644)).
- Re-suspend each cell pellet with 1 mL of IPM for DG, 2 mL of IPM for SVZ, and then plate cells into one well of a 24-well tissue culture plate (CLS3527) for DG, and 2 wells for SVZ cells. Incubate cells in the CO2 incubator for 48 h.
- Change half the IPM, avoiding removing any cells. Continue to incubate cells for 7–14 days, changing half the IPM every other day and monitoring the cells for the formation of neurospheres. Neuropheres should form in both cultures in 1–2 weeks.
Differentiation of Human iPSCs into NSCs
Dual SMAD inhibition is a well-established method to derive neural progenitor cells from human ES/iPS cells in monolayer cultures. This protocol uses two SMAD inhibitors, Noggin (SRP4675) and SB431542 (S4317), to drive the rapid differentiation of ES/iPS cells into a highly enriched population of NPCs. Noggin acts as a BMP inhibitor and SB431542 inhibits the Lefty/Activin/TGFβ pathways by blocking the phosphorylation of ALK4, ALK5, and ALK7 receptors. In an effort to make a more defined and optimized neuronal differentiation protocol, Li and colleagues modified the original protocol to establish a completely small molecule-based differentiation method, which relies on three small molecules to inhibit GSK-3β, CHIR99021 (SML1046), TGFβ, SB431542 (S4317), and Notch, Compound E (565790) signaling pathways, along with human LIF (LIF1010). This new small molecule-based neural differentiation protocol increased neural differentiation kinetics and allowed the derivation of truly multipotent neural stem cells that respond to regional patterning cues specifying forebrain, midbrain, and hindbrain neural and glial subtypes.
Application Note: Robust Differentiation of Human iPSCs into Lineage-Specific Neuronal and Glial Cells Utilizing Dual-SMAD Inhibition
Characterization of Neural Stem Cells
Presently, neural stem cells are often identified based upon the presence of molecular markers that are correlated with the stem and/or progenitor state along with the absence of a more differentiated phenotype as assessed through marker analysis. NSCs positively express stem cell markers Nestin (ABD69, MAB353), Sox-2 (AB5603) and Musashi (MABE268) and lack the more differentiated lineage markers including βIII-tubulin (MAB1637) for neurons, GFAP (AB5804) for astrocytes and O1 (MAB344) for oligodendrocytes.
Expansion of Neural Stem Cells
Culture of NSCs in 3D Neurosphere Culture
- After 7–12 days of neurosphere culture (step 1-15 above), collect all primary spheres without disturbing the attached cells; spin at 200 g for 5 min at room temperature.
- Carefully remove the medium and add 1 mL of 0.05% trypsin/EDTA (59417C) to each tube. Dissociate the spheres using a 1-mL blue tip by pipetting up and down 20 times to digest the spheres within 2 min. Add 1 mL of Trypsin Inhibitor Solution (T7659) to each tube and pipette up and down 10 more times. Add 5 mL of IPM to each tube, mix by inverting the tubes a few times, and pellet the cells at 200 g for 5 min at room temperature.
- Re-suspend the DG cell pellet in 1 mL of IPM and the SVZ cell pellet in 2 mL of N2 medium. Dilute a 10-µl aliquot from each sample in 10 µL of 0.5% Trypan blue (T8154) and count viable cells using a hemocytometer.
- Plate the DG cells into one well of a 24-well plate, and the SVZ cells into one well of 6-well plate and incubate cells.
- Replace half the medium with fresh IPM for the DG cells and fresh N2 medium for the SVZ cells every other day for 1 week. Avoid taking any cells out when changing out half the medium. After the first passage, maintain the DG and the SVZ NSCs in N2 medium and passage NSCs by seeding at 3X104 -1X105 cells/mL every 2–3 days.
Culture of NSCs in 2D Monolayer Culture
We recommend coating tissue culture plastic or glassware with poly-L-ornithine and laminin. Poly-L-ornithine and laminin provide optimal matrix for adhesion and growth of the NSCs.
- Dilute poly-L-ornithine (P4957) with water from the stock concentration (0.1 mg/mL) to yield: a. 20 μg/mL for polystyrene plates b. 50 μg/mL for glass plates.
- Add enough of the poly-L-ornithine solution to cover the whole surface of the tissue culture-ware. Use 2 mL volume for 3.5 cm plates, 5 mL volume for 6 cm plates and 10 mL volume for 10 cm plates and T75 flasks. Incubate in a humidified 37 °C incubator for at least one hour.
- Remove the poly-L-ornithine solution and rinse once with sterile water. Aspirate after the rinse.
- For the culture, propagation and differentiation of NSCs, dilute laminin (L2020) to final concentration of 5 μg/ml. Use 2 mL volume for 3.5 cm plates, 3-5 mL volume for 6 cm plates and 7-10 mL volume for 10 cm plates and T75 flasks. Incubate in a humidified 37C incubator for at least 1 hour. Coated plates and flasks can be stored in the laminin solution at 2-8 °C for 3 weeks or at -20 °C for 6-8 months. Just before use, bring the coated plates or flasks up to room temperature and aspirate the laminin solution. Rinse the plates once with 1X PBS (806552) before use.
- Remove the vial of Neural Progenitor Cells (SCC007, SCC008, SCR055, SCC035) from liquid nitrogen and incubate in a 37 °C water bath. Closely monitor until the cells are completely thawed. Maximum cell viability is dependent on the rapid and complete thawing of frozen cells.
IMPORTANT: Do not vortex the cells.
- As soon as the cells are completely thawed, disinfect the outside of the vial with 70% ethanol. In a laminar flow hood, use a 1 or 2 mL pipette to transfer the cells to a sterile 15 mL conical tube. Be careful to not introduce any bubbles during the transfer process.
- Using a 10 mL pipette, slowly add dropwise 9 mL Neural Expansion Medium (SCM005, SCM004) (pre-warmed to 37 °C) to the 15 mL conical tube.
IMPORTANT: Do not add the whole volume of medium at once to the cells. This may result in decreased cell viability due to osmotic shock.
- Gently mix the cell suspension by slow pipetting up and down twice. Be careful to not introduce any bubbles.
IMPORTANT: Do not vortex the cells.
- Centrifuge the tube at room temperature at 200 x g for 3-5 minutes to pellet the cells.
- Decant as much of the supernatant as possible.
- Resuspend the cells in a total volume of 2 mL of Neural Expansion Medium (prewarmed to 37 °C) containing FGF-2, 20 ng/mL (F0291).
- Plate the cell mixture onto a poly-L-ornithine and laminin-coated 3.5 cm tissue culture plate.
IMPORTANT: For optimal growth, thawing the cells on tissue culture plates that are larger than a 3.5 cm tissue culture plate is not recommended.
- Incubate the cells at 37 °C in a 5% CO2 humidified incubator.
- The next day, exchange the medium with fresh Neural Expansion Medium (prewarmed to 37 °C) containing FGF-2 (20 ng/mL). Exchange with fresh medium every other day thereafter.
- When the cells are approximately 90 - 100% confluent, they can be dissociated with Accutase™ (A6964) and passaged or alternatively frozen for later use. The cells should be maintained at high cell density at all times and thus the recommended passaging is at 1:2 to 1:6.
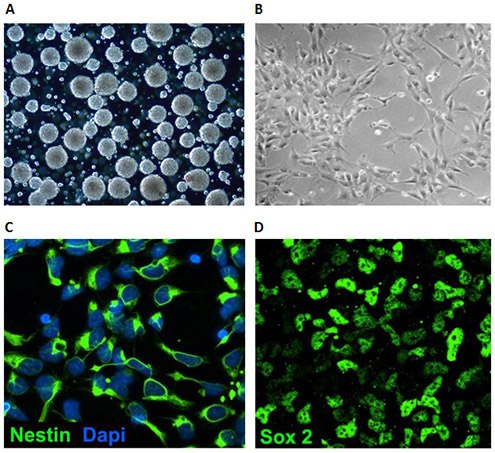
Figure 2. Neural stem cell culture characteristics. NSCs can be grown as floating 3D neurospheres (A) or attached 2D monolayers (B) on poly-L-ornithine/laminin coated plates. Multipotent neural stem cells express Nestin (C) and Sox-2 (D).
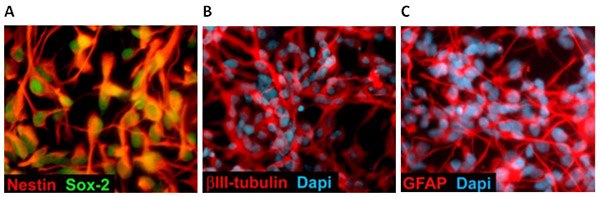
Figure 3. Differentiation of Neural Stem Cells. Multipotent NSCs express Nestin/Sox-2 and can differentiate into BIII-Tubulin positive neurons (B) or GFAP positive astrocytes (C) under appropriate culture conditions.
Frequently Asked Questions
- What is the doubling time of NSCs? How often do I split cultures?
Human NSCs double every 48-72 hours. Rodent NSCs double every 24 hours. Cells should be split 1:2 to 1:6 once 80% confluent, roughly every 3-5 days. - My cells do not adhere to the P/L coated plates, what can I do to establish a monolayer NSC culture?
The Poly-L-Ornithine/Laminin coated plates are faulty. Recoat new plates with fresh Poly-L-Ornithine/Laminin solutions - My cells are growing slowly, what went wrong?
Change the media every day to ensure fast proliferation. Use fresh media with freshly supplemented bFGF. Wait until the cells are 80-90% confluent before splitting at 1:2 to 1:4 split ratio. Check to make sure cells have not differentiated. - There is a lot of cell death/floating cells in my culture. Is this normal?
This is a loosely adherent cell culture, and it is not uncommon to have cells and cell debris floating in the culture media. As long as the adherent cells on the plate are growing nicely in a monolayer, do not worry about the floating cells and they will be washed away during media changes. - Do I have to plate these cells at such a high density or can I split them 1:3 or 1:4?
Unlike most cell culture systems, these cells need to be plated densely to be highly proliferative. Proliferation is slowed in lower density cultures presumably due to a loss of cell to cell contact. Passaging the cells at a 1:2 ratio is ideal, however if the cells are growing nicely it is possible to split the cells 1:3. - How do you differentiate the cells into specific neural phenotypes?
The differentiation protocol/media provided is intended as a basal system. For specific cell types, differentiation protocols including additional neurotropic factors can be developed as determined by the end user’s interests.
Ordering Information
Neural Stem Cell Lines
Neural Stem Cell Media and Supplements
Neural Stem Cell Culture Reagents
Neural Stem Cell Markers
To continue reading please sign in or create an account.
Don't Have An Account?