3D Neurosphere Culture Media
Brain cancers of the the central nervous system include neuroblastomas, gliomas, meningiomas and pituitary adenomas. One of the most aggressive brain tumors are astrocyte-derived glioblastoma multiforme, of which the U-87 MG glioblastoma cell line (89081402) was derived. The 3-dimensional neurosphere cell culture technique was first described by Reynolds and Weiss (1992) (9) and involves the culture of neural stem cells in freely-floating spherical clusters termed neurospheres. Neurospheres from brain-derived tumors are thought to develop from cancer stem cell (CSC) subpopulations within the tumor when grown in a 3D environment. These cancer stem cell populations express stem cell related markers Nestin and Sox2 while lacking GFAP. The 3D culture of brain-derived cancer cells is considered to be a more biologically relevant cell culture model maintaining the original malignant tumor phenotype.
Adherent brain cancer cells cultured in fetal bovine serum (FBS) undergo unwanted phenotype changes and cell differentiation, while culturing in 3D neurospheres in serum-free conditions maintains the original properties of the primary tumor (1). However, conventional serum-free media for culturing neurospheres struggle to efficiently maintain the self-renewing subpopulations of cancer stem cells (CSCs). Consequently, the stem cell population is gradually lost overtime along with the cell proliferation rates. The PromoCell 3D Tumorsphere Media XF (C-28070, C-28075) is designed to serially passage brain tumor derived cell lines as undifferentiated 3D neurospheres with high cell proliferation rates. It’s defined and serum-free formualtion allows consistent and standardized expansion of neurospheres while maintaining the cancer stem cell characteristics such as self-renewal and chemoresistance.
Neurosphere Culture Protocol
- Harvest the adherent cells. Harvest the neural cells as a single cells suspension and resuspend in 3-5 mL of fresh 3D Tumorsphere Media XF media (C-28070, C-28075).
- Count the cells. Count viable cells and spin down cells at 300 x g for 5 minutes. Obtain a concentration of 1X106 cells/ml.
- Seed the cells. Seed the cells in appropriate suspension culture vessel (Corning ULA or Spheroid Plates) at 1X104 cells/ml in each well of a 6-well plate (ie. 4X104 cells in 4 ml).
- Allow cells to grow. Incubate for 4-10 days. Refresh half the media with fresh 3D Tumorsphere Media XF (C-28070, C-28075) media every 3-4 days.
- Passage the neurospheres. Passage the neurospheres before they develop a dark necrotic center. Transfer all the neurospheres to a 15 ml conical tube. Allow the neurospheres to settle by gravity for 10-15 min at room temperature.
- Wash the spheroids. Remove supernatant and wash 2X’s with PBS (D8537) following the same step as #5 above.
- Enzymatically treat neurospheres. Aspirate PBS and resuspend in 1 ml Trypsin-EDTA (T3924) and incubate for 2-4 minutes at room temperature. Pipette up and down every 30 seconds. Do not allow neurospheres to settle.
- Break apart the neurospheres. Pipette up and down 10-20 times with a P1000 to break apart the neurospheres into smaller aggregates. Add twice the volume of Trypsin Neutralization Solution (T6414).
- Count the cells. Count viable cells and spin down cells at 300 g for 5 minutes. Resuspend cells at 1 million cells/ml in fresh 3D Tumorsphere Media XF media (C-28070, C-28075).
- Seed the cells. Using a low attachment plate seed 1X104 cells/ml in each well of a 6-well plate (ie. 4X104 cells in 4 ml media).
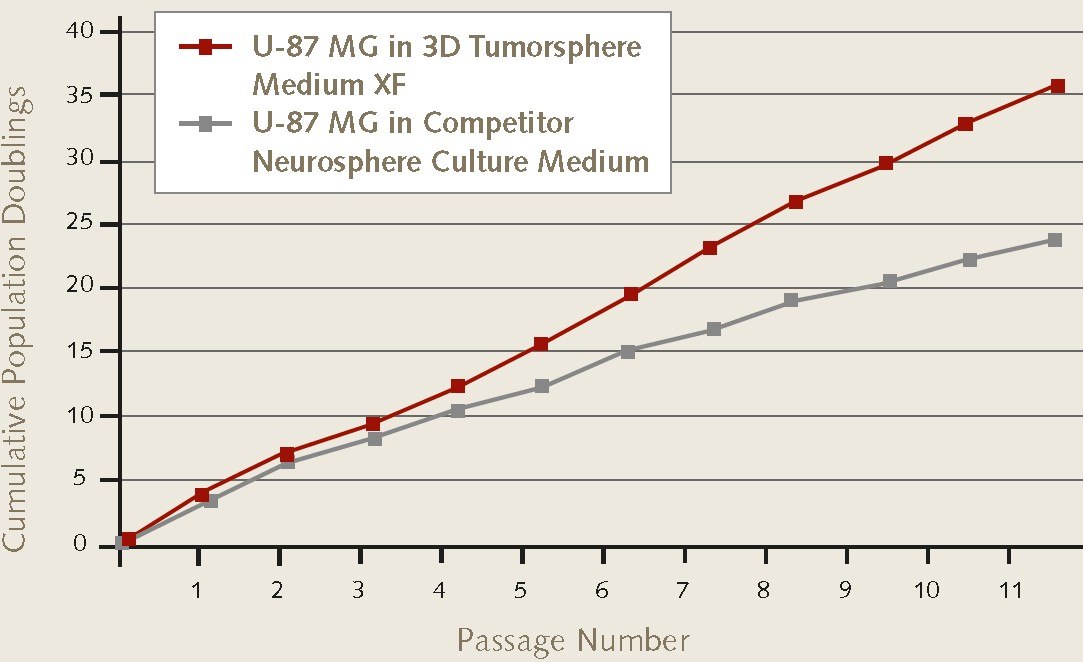
Figure 1. Cell proliferation rates of U-87 MG glioblastoma cells during 3D neurosphere culture.Neurosphere proliferation rates of U-87 MG glioblastoma cells (89081402) were higher over 11 passages in Promocell’s 3D Tumosphere Media XF vs. other competitior neurosphere culture media.
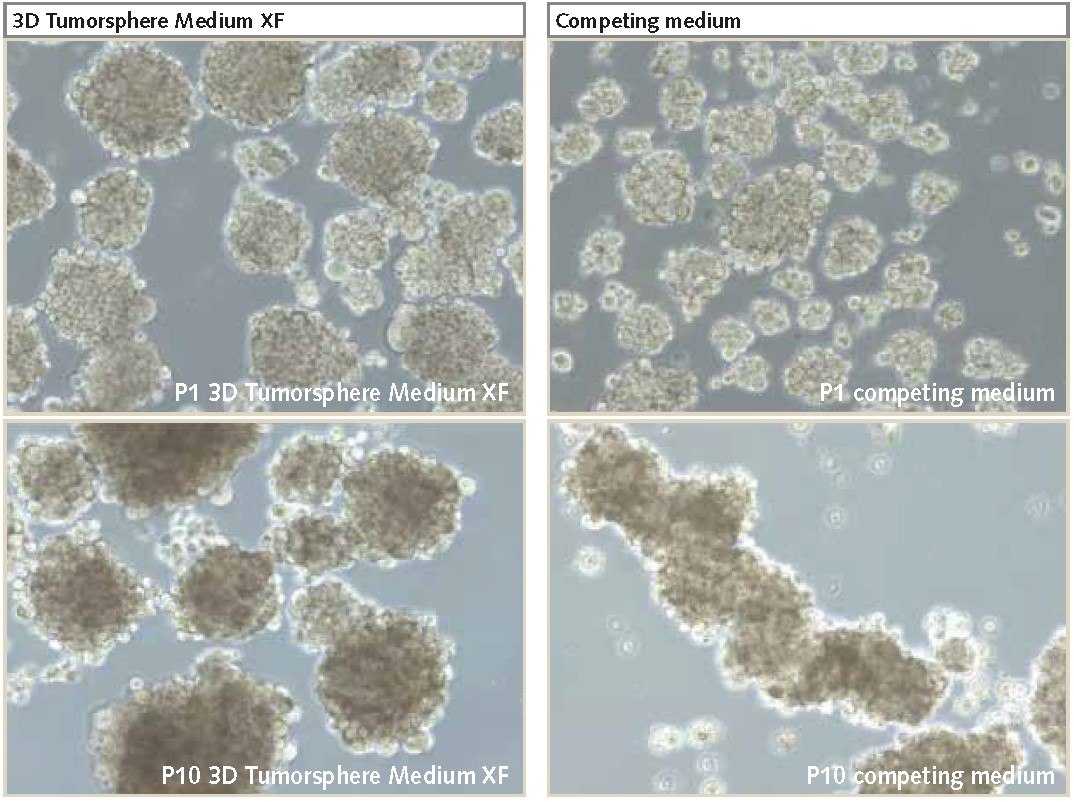
Figure 2. U-87 MG cells cultured as 3D neurospheres.Neurospheres cultured in PromoCell’s 3D Tumorsphere Media XF produces higher proliferation rates and larger neurospheres over multiple passages (P10) compared to competitor products (100X magnification).
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?