Using Immortalized 16HBE14o- Human Bronchial Epithelial Cell Lines to Model Respiratory Lung Diseases
- What are 16HBE14o- Cells?
- Human Lung Epithelial Cell Line Collection
- Air-Liquid Interface (ALI) 3D Cell Cultures
- Tips & Tricks for Performing 16HBE14o- ALI using Millicell® Standing Cell Culture Inserts
- Cell Model of Cystic Fibrosis and CFTR Function
- Respiratory Viral Disease Mechanisms (H1N1, SARS-CoV, COVID-19)
- Asthma and COPD Research
- Exposure to Air Pollution
- Smoking and Vaping Effects on Lung Function
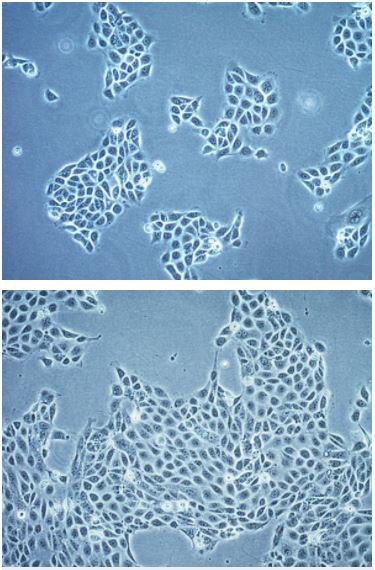
Figure 1.The 16HBE14o- cell line is a suitable model for studying molecular mechanisms that retains characteristic features of normal differentiated bronchial epithelial cells.
What are 16HBE14o- cells?
Bronchial epithelial cells make up the functional barrier along the main passageways into the lungs, constituting a critical line of defense against respiratory pathogens. Physiological models are essential for understanding the interaction between respiratory diseases and epithelial cells. Primary cells and cell lines cultured at the air-liquid interface (ALI) closely represent native cells, preserving key characteristics of in vivo airway epithelium targeted by viruses.
The 16HBE14o- human bronchial epithelial cell line (SCC150) was isolated from the bronchus of a heart-lung patient and immortalized with pSVori-, an origin-of-replication-defective SV40 plasmid. This cell line is a suitable model for studying molecular mechanisms and expresses high levels of cystic fibrosis transmembrane conductance regulator (CFTR) mRNA and protein. 16HBE14o- retains characteristics of normal differentiated bronchial epithelial cells including:
- Cobblestone morphology
- Cytokeratin expression
- Ability to form tight junctions (TJs)
- Directional ion transport
- Ability to generate transepithelial electrical resistance (TEER)
- Functional cilia detectable when cultured at ALI
Human Lung Epithelial Cell Line Collection
We offer a complete collection of human lung epithelial cell lines licensed from the Gruenert Lab and housed at the NIH Cystic Fibrosis Research Center at the University of California, San Francisco. These extensively-published human cell lines are valuable models for pulmonary diseases such as cystic fibrosis, asthma, chronic obstructive pulmonary disease (COPD), and lung cancer. They also provide relevant in vitro models for researching the consequences of exposure to air pollution, smoking and vaping, and modeling respiratory viral infections such as influenza H1N1, SARS-CoV, MERS-CoV, and COVID-19.
Air-Liquid Interface (ALI) 3D Cell Cultures
Air-liquid interface (ALI) 3D cell culture techniques support the differentiation of human bronchial epithelial cells into mature lung phenotypes from cell lines such as 16HBE14o-. Bronchial epithelial cells cultured using ALI cell culture techniques can form polarized cell layers and tight junctions, can differentiate, and present functional cilia. ALI cell culture methods rely on Millicell® cell culture inserts to support the development of the pseudostratified mucociliary phenotype observed in vivo. 16HBE14o- ALI References
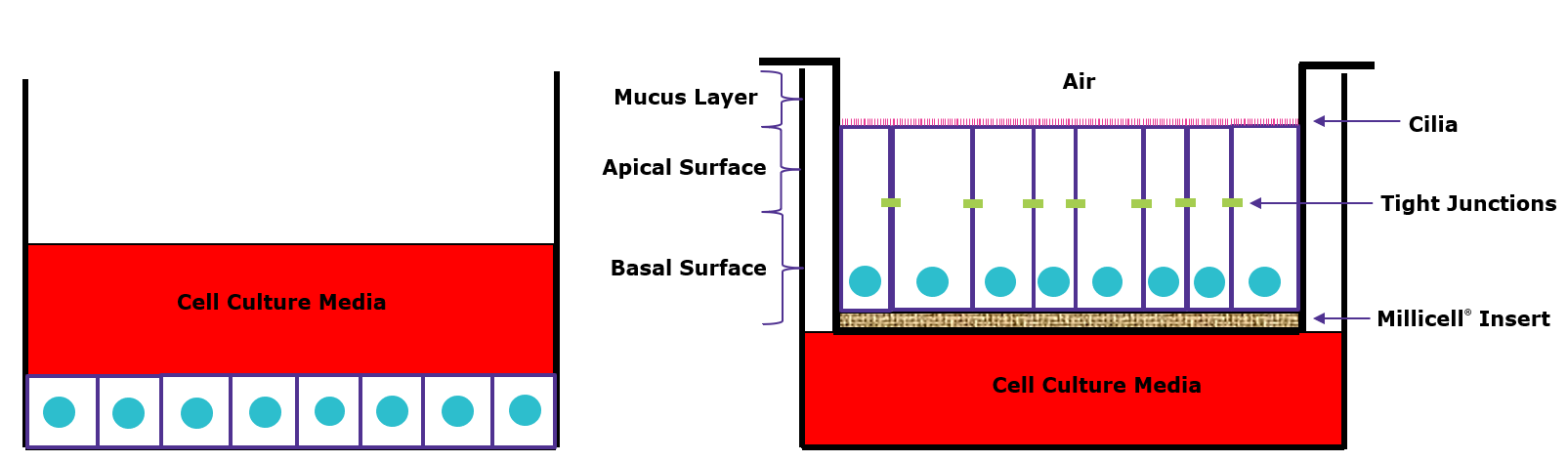
Figure 2.Airway epithelial cells cultured in traditional 2D (left) vs. 3D air-liquid interface (ALI) (right). In traditional 2D culture, the culture media is added on top of the cells so they are not exposed to air. In 3D ALI culture, the cells are cultured on inserts and only the basal surface is exposed to media while the apical side is exposed to air to better represent the in vivo structure.
Air-Liquid Interface Cell Culture Protocol
- Coat tissue culture flasks with ECM mixture: Culture and expand 16HBE14o- cells on ECM coated flasks in ∝-MEM (M2279), 10% FBS (ES-009-B), 2 mM L-Glutamine (TMS-002-C) and 1X Penicillin-Streptomycin Solution (TMS-AB2-C). Do not let cells become overconfluent and split after reaching 90-95% confluence. Remove adherent cells with Trypsin-EDTA solution (T3924).
- Seed 16HBE14o- cells into a Millicell® hanging cell culture insert (PTHT12H48) at a density of 105 cells/cm2 in 1-2 mL expansion media listed in step 1 above. Submerge cells for 24-hours in an incubator at 37 °C. For protcols using Millicell® standing inserts, see Tips & Tricks below.
- Initiate the air-liquid interface culture, transferring the culture insert/cells into a new 12-well plate (CLS353043). The entire culture media within the insert can be removed, exposing cells to air. Change the media on the basolateral side of the insert every day.
- On or after day 8, cells can be analyzed using common cell-based assays or antibody staining.
Tips & Tricks for Performing 16HBE14o- ALI using Millicell® Standing Cell Culture Inserts
This protocol can also be performed on Millicell® standing cell culture inserts. The 0.4m polycarbonate membrane insert (PIHP01250) is optically translucent, but cells can be imaged by removing the membrane from the insert with a no. 11 scalpel blade. The 0.4 µm hydrophilic PTFE membrane (PICM01250) is optically transparent and can be imaged directly from the insert.
- The day prior to seeding cells, place Millicell® standing inserts into 24-well Falcon® cell culture plates (CLS353047) and coat the apical side of the membrane (PIHP01250) with 150 µL ECM mixture. For hydrophilic PTFE membranes (PICM01250), we recommend 60 µL of diluted collagen (collagen/ethanol mixture).
- Place coated inserts into a new 24-well plate and seed 200 μL 16HBE14o- cells (1.33 x105 cells/cm2) on the apical side. Add 400 µL media to the basolateral side. Add an additional 200 µL media to the apical side the following day.
Note: We recommend using the Millicell® DCI to monitor cell growth on the transparent PTFE membrane (PICM01250) in the first 4 days of cell culture. Full coverage should be observed before starting ALI. - Four days after seeding cells, start Day 0 of ALI by removing media from the apical side, exposing the cells to air. Reduce basolateral media volume to 250 µL to prevent floating.
- Exchange the basolateral media every other day for up to 14 days.
- Measure TEER every other day starting on Day 0 of ALI. Fix cells with 4% formaldehyde (47608) for confocal imaging and immunostaining on Days 1, 8 and 14 of ALI. Perform lucifer yellow (LY) permeability assays on Days 0, 8 and 14 of ALI. To measure TEER and perform LY assays, place inserts in a new 24-well plate and add fresh room temperature media:
- For TEER, add 400 µL media in the apical side and 600 µL in the basolateral side. It’s best practice to measure TEER as soon as possible after adding the apical volume.
- For LY assays, add 400 µL media in the apical side and 400 µL in the basolateral side.
Cell Model of Cystic Fibrosis and CFTR Function
Cystic fibrosis is a hereditary disease affecting multiple organ systems, including the lungs and digestive system. This disorder is characterized by abnormalities in mucus-producing glands, resulting in secretions disrupting airways with thick and sticky mucus that clogs the lungs, and can lead to death. The cystic fibrosis transmembrane conductance regulator (CFTR) protein helps to maintain the balance of salt and water within the body, including the surface of the lungs. Mutations or absence of the CFTR gene can lead to cystic fibrosis.
The 16HBE14o- cell line (SCC150) is a wild-type airway epithelial cell line, and expresses high levels of CFTR mRNA and protein. The parental CFBE41o- cell line (SCC151) is derived from a CF patient homozygous for the ΔF508 CFTR mutation. Subsequent clones of this line have been created with the ΔF508 CFTR mutation corrected (SCC158-SCC161).
Andersson C, Al-Turkmani MR, Savaille JE, Alturkmani R, Katrangi W, et al. 2008. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J Lipid Res. 49(8):1692-700
Muir A, Soong G, Sokol S, Reddy B, Gomez MI, et al. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 30(6):777-83.
Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, et al. 2010. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 12(9):863-75.
16HBE14o- Cystic Fibrosis References
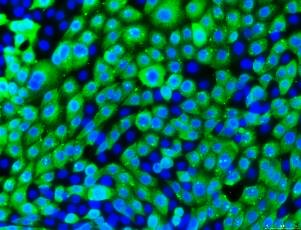
Cytokeratin 18
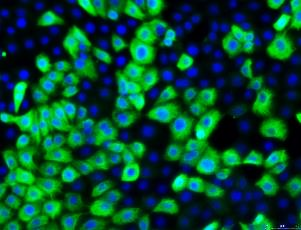
Pan-Cytokeratin
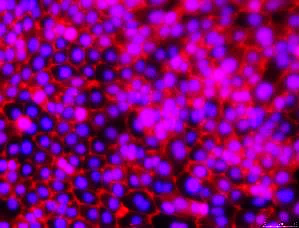
ZO-1 Tight Junctions
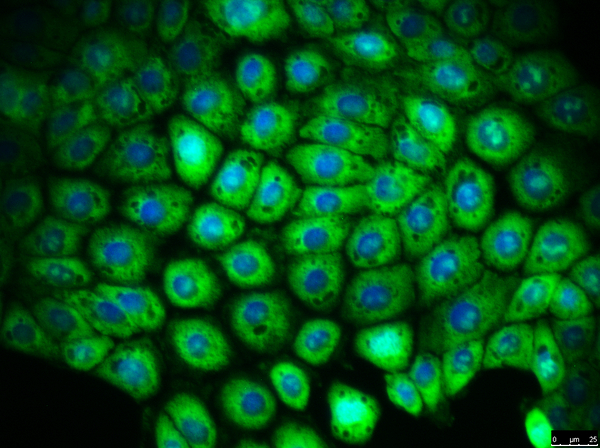
CFTR (WT)
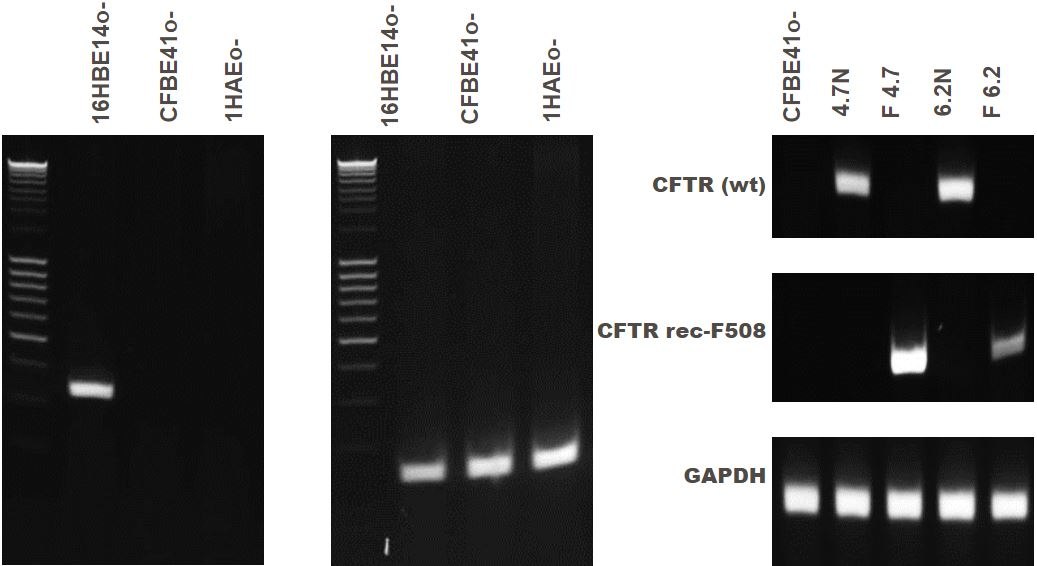
Figure 5. CFTR gene expression of bronchial epithelial cell lines.16HBE14o- cells express wild-type CFTR while CFBE41o- and 1HAEo- lack CFTR gene expression. CFBE41o- cells lines are derived from a cystic fibrosis patient that does not express wild-type CFTR. Four clones have been generated with the mutation corrected (4.7N, delta F4.7, 6.2N and delta F6.2).
Respiratory Viral Disease Mechanisms (H1N1, SARS-CoV, COVID-19)
Bronchial epithelial cells make up the functional barrier along the main passageways into the lungs, constituting a critical line of defense against respiratory pathogens (including influenza H1N1, SARS-CoV, MERS-CoV, RSV and COVID-19). The 16HBE14o- cell line has been used to study and characterize numerous viral infection mechanisms to support research for vaccine and drug development. For example, 16HBE14o- cells express angiotensin-converting enzyme 2 (ACE2) and the serine protease TMPRSS2 which play an essential role in SARS-CoV infection and viral replication.
Kam YW, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R. 2009. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One. 4(11):e7870.2
Wu XL, Ju DH, Chen J, Yu B, Liu KL, He JX, et al. 2003. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr Microbiol. 67(4):431-6.
Qin L, Peng D, Hu C, Xiang Y, Zhou Y, et al. 2014. Differentiation of Th subsets inhibited by nonstructural proteins of respiratory syncytial virus is mediated by ubiquitination. PLoS One. 9(7):e101469.
16HBE14o- Virus References
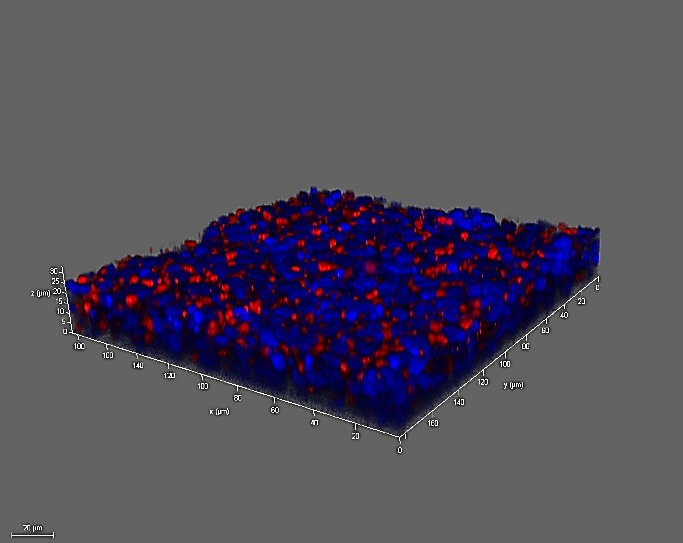
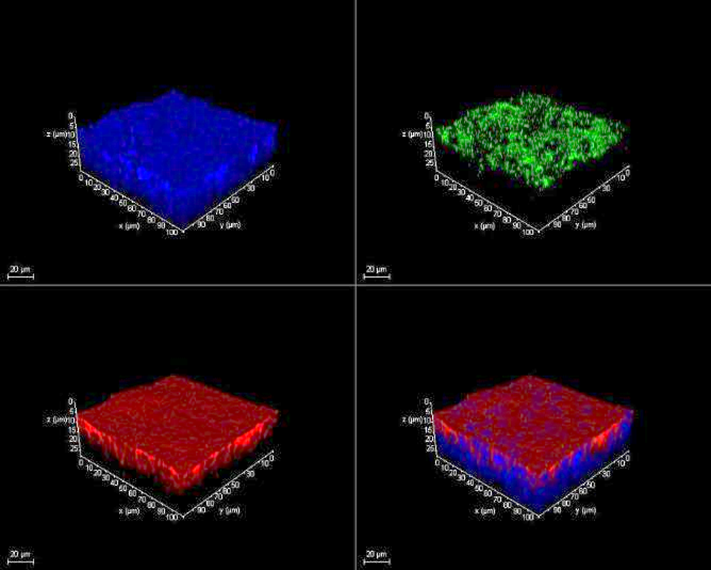
ACE2 DAPI
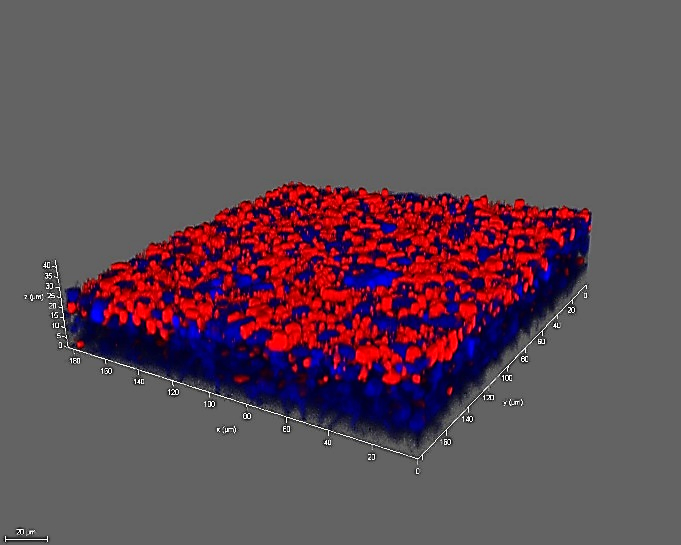
TMPRSS2 DAPI
Figure 6. 16HBE cells express ACE-2 and TMPRSS2. Left to right: 16HBE14o- cells cultured using the air-liquid-interface (ALI) technique for 8 days show expression of angiotensin-converting enzyme 2 (ACE2) and the serine protease TMPRSS2 which play an essential role in SARS-CoV (COVID-19) infection and viral replication.
Cell Models for Asthma and COPD Research
Bronchial asthma and chronic obstructive pulmonary diseases (COPD) are obstructive pulmonary diseases characterized by underlying airway inflammation, which can lead to breathing difficulties, mucus overproduction and lung damage. 16HBE14o- cell lines have been used in cell models to study inflammatory cell recruitment, activation and basic mechanisms to study asthma and COPD lung diseases.
Hackett TL, de Bruin HG, Shaheen F, van den Berge M, van Oosterhout AJ, et al. 2013. Caveolin-1 controls airway epithelial barrier function. Implications for asthma. Am J Respir Cell Mol Biol. 49(4):662-71.
Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, et al. 2013. Barrier disrupting effects of Alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 8(8):e71278.
Pace E, Ferraro M, Minervini MI, Vitulo P, Pipitone L, et al. 2012. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One. 7(3):e33601.
16HBE14o- Asthma/COPD References
Exposure to Air Pollution
Exposure to toxic air pollutants such as ozone (O3), benzenes, and nitrogen dioxide (NO2) from vehicle exhaust emissions and burning liquid petroleum gas play important roles in the development of allergic airway diseases and lung cancer. 16HBE14o- cell lines have been used in cell models to study the damaging effects of air pollution on the human bronchial epithelium.
Jiang CL, He SW, Zhang YD, Duan HX, Huang T, et al. 2017. Air pollution and DNA methylation alterations in lung cancer: A systematic and comparative study. Oncotarget. 8(1):1369-1391
Zhou Z, Liu Y, Duan F, Qin M, Wu F, et al. 2015. Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells. PLoS One. 10(9):e0138267.
Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. 2002. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 18(5):315-20.
16HBE14o- Air Pollution References
Smoking and Vaping Effects on Lung Function
Damage from smoking traditional cigarettes and e-cigarettes (vaping) has been shown to increase instances of epithelial inflammation and injury and to disrupt host defense functions of airway epithelium. Additionally, cigarette smoke can impair airway epithelial barrier function and contribute to lung cancer. 16HBE14o- cell lines have been used in cell models to study the damaging effects of cigarette smoking and vaping on human airway epithelial cells.
Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. 2012. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 39(2):419-28.
Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, et al. 2007. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. (6):748-55.
Gerloff J, Sundar IK, Freter R, Sekera ER2, Friedman AE. 2017. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol. 3(1):28-40.
16HBE14o- Smoking/Vaping References
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?