Versatile Cell Culture Scaffolds: Bio-orthogonal Click
Devising biomaterial scaffolds that are capable of recapitulating critical aspects of the complex extracellular nature of living tissues in a three-dimensional (3D) fashion is a challenging requirement in the field of tissue engineering and regenerative medicine. Hydrogels are one class of materials that have been widely studied and developed as such synthetic extracellular matrix mimics (ECM). Hydrogels, due to their high water content, elasticity, and transport properties, are suitable for culturing cells in 2D and 3D environments, and simulate some of the critical mechanical aspects of soft tissue environments.1-5
From a basic perspective, hydrogels can be fabricated from a wide range of natural and synthetic precursors that crosslink via physical, ionic, or covalent interactions.2 When cells are cultured in such systems, hydrogels formed from natural polymers provide a plethora of biological signals (e.g., cell adhesion proteins, depot of growth factors, proteolytically degradable domains), but often lack flexibility in controlling physicochemical properties of the final material in a reproducible manner. Alternatively, hydrogels formed from synthetic macromolecules allow precise engineering of critical properties, such as mechanics, swelling, and degradation, but typically use chemistries that are unrecognizable to cells. Thus, there is a growing interest to develop strategies to synthesize gels as cell delivery vehicles and culture systems that integrate the benefits of both synthetic and naturally derived materials. The perspective provided herein will discuss some of these concepts in the context of poly(ethylene glycol) (PEG)-based hydrogels inherently. PEG hydrogels have been widely used for 2D and 3D cell culture, and various strategies have been employed to control PEG gel properties and integrate cellular cues.3-5 Of further significance is that PEG materials minimize non-specific protein interactions, which enable researchers to better understand how cells receive specific signals from their extracellular environment.
Chemistry and Molecular Design Principles for PEG Hydrogel Synthesis
PEG hydrogels have been formed using linear or branched/star polymeric chains that are either covalently or non-covalently crosslinked to each other, typically under aqueous conditions. Physically crosslinked block copolymers of PEG and poly(propylene oxide) (e.g., Pluronics) have enjoyed widespread use as in situ forming hydrogels with lower critical solution temperature (LCST) tuned around 37 °C;6 however, these noncovalently assembled hydrogels have a limited range of mechanical properties. As an alternative, covalent crosslinking methods have become an indispensable tool for construction of highly stable PEG hydrogels.2 To build covalently crosslinked networks for cell culture, one must also be mindful of the cytocompatibility of the crosslinking method. In this work, two polymerization mechanisms have been widely used: (i) chain growth and (ii) step-growth.4,5 Chain polymerization of end-functionalized PEGs is robust and simple,4 but results in a complex network structure that is structurally heterogeneous with a broad distribution of kinetic chain lengths and degradation that is limited to the network crosslink points. More recently, step-growth polymerization of PEGs has emerged as a facile method to achieve controlled and more uniformly crosslinked polymeric networks, while simultaneously providing a simple route to introduce biological signals. Typically for a PEG based step-growth hydrogel, a multi-functionalized molecular system is crosslinked using bi-functionalized molecules in a stoichiometric manner; in general, the average functionality must be greater than two to form a gel. Depending upon the required mesh size or crosslinking density, any one of these molecules or both can be derived from a PEGbased polymer (Figure 1). For hydrogels aimed at 3D cell culture and tissue regeneration, physiological conditions, and highly efficient, nontoxic chemical crosslinking methods are the critical requisites since gels often have to be formed in the presence of cells and proteins. These complexities have prompted bioengineers to explore a variety of chemical reactions for step-growth hydrogel formation that are mild and specific, but still proceed at an appropriate rate under physiological pH and temperature.

Figure 1.Structures of 4-arm and linear PEG precursors and a schematic illustration of the resulting step-growth hydrogel formed using these PEG macromolecules.
Click Reactions in the Development of Complex, Yet Well-defined Scaffolds
One of the recent chemical strategies that has brought significant advances to the field of biomaterials is ′click chemistry′, introduced by Sharpless and co-workers to describe reactions that are rapid, highly selective, and high yielding in connecting two molecular components together under aqueous conditions.7 While copper catalyzed azidealkyne cycloaddition (CuAAC) (Figure 2A) was the first to be recognized as a click reaction, a variety of chemical reactions, including Michael additions (Figure 2B), photo-initiated thiol-ene reactions (Figure 2C), Staudinger ligation, and strain promoted azide-alkyne cycloaddition (SPAAC), have been later identified to fulfill the concept of click chemistry.7-9 Since this group of reactions is not only orthogonal to each other, but also orthogonal to functional groups in biological systems, they have emerged as superior chemical tools for biomaterial scientists to construct complex material scaffolds that are well-controlled and highly defined.
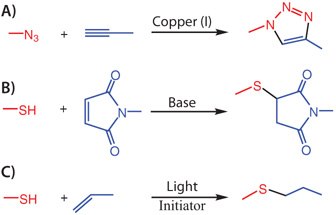
Figure 2.Popularly known click reactions: A) Copper catalyzed azide-alkyne cycloaddition; B) Base catalyzed Michael addition reaction between thiol and maleimide; C) Photoinitiated thiol-ene coupling.
With the above introduction to PEG hydrogels and click chemistry, the remaining sections of this article will focus on: (i) the utilization of various click reactions in constructing PEG hydrogels for cell culture applications and (ii) how the orthogonality of click reactions has been exploited to precisely functionalize these materials with selective biological epitopes to direct cellular functions.
Michael Additions
Michael-type additions are attractive click reactions to form hydrogel materials in the presence of cells, owing to their mild reaction conditions and favorable reaction rates. This type of reaction proceeds due to the nucleophilic attack of thiolate anion (Michael donors) on electron deficient double bond of α, β-unsaturated ketones (Michael acceptors) as shown (Figure 2B). Vinyl sulfones, maleimides, and acrylates are widely used Michael acceptors to form hydrogels. Hubbell and co-workers10,11 were one of the first to develop step-growth hydrogels using 4-arm PEG tetra vinyl sulfone and cysteine flanked peptides (Figure 3) for cell culturing applications. Since de-protonation of the thiol to the thiolate anion is critical for these reactions and requires addition of base, Hubbell and co-workers circumvented this issue by simply adjusting the pH of the buffer. This synthetic route allowed them to incorporate cysteine-containing peptides without requiring additional modification. By this route they demonstrated numerous peptide epitopes to control cell adhesion (e.g., Arg-Gly-Asp-Ser (RGDS), Ile-Lys- Val-Ala-Val (IKVAV)), as well as a plasmin sensitive peptide crosslinker that allowed degradation by cell-secreted proteases. Recently, Garcia and co-workers12 reported maleimide functionalized multi-arm PEGs with faster reaction kinetics using very low concentrations of base to crosslink the di-thiol peptide crosslinkers, which provides additional benefits when encapsulating more sensitive cell types.

Figure 3.Step-growth hydrogel formation between 4-arm PEG tetra vinyl sulfone and cysteine end functionalized MPP cleavable peptide via Michael addition.
Thiol-ene Chemistry
Unlike Michael addition reactions (i.e., anionic), thiol-ene reactions proceed via a radical mechanism in which a thiyl radical can add over to either an electron deficient or electron rich carbon-carbon double bond (termed -ene).8 A typical thiol-ene mechanism involves (Figure 4A): (i) abstraction of a hydrogen atom from a thiol by an initiating radical, (ii) the resultant thiyl radical adding over to an alkene functionality creating a carbon radical, and (iii) chain transfer of the carbon radical to another thiol, regenerating a thiyl radical which then propagates to a new alkene. While the thiyl radicals can be generated from thermal, photo, and redox initiators, photoinitiators are especially attractive for cell relevant applications, as cytocompatible photoinitiation conditions have been widely reported8 and also allow the user to control the reaction spatially and temporally.
Our group first reported the synthesis of peptide-functionalized PEG hydrogels using photoinitiated thiol-ene chemistry.13 In our approach (Figure 4), norbornene-functionalized multi-arm PEGs and cysteine containing chymotrypsin cleavable peptides were crosslinked to create PEG hydrogels, using lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as a water-soluble photoinitiator and 365-420 nm light in PBS buffer under physiological pH conditions. Both gel forming conditions and the resultant hydrogel system supported high levels of cell viability (>95%). More importantly, because this chemistry is based on light, photopatterning of cell signals, such as the cell-adhesive RGDS, at specific locations of the gel was achieved (Figure 4), which ultimately dictated local cell interactions and morphology. This facile incorporation of any thiolated cell signaling moieties (e.g., thiolated proteins) on hydrogel scaffolds post cell encapsulation enables experimenters the possibility of understanding and directing cellular functions (e.g., differentiation) in a spatio-temporal fashion. In a similar line of inquiry, thiol-acrylate polymerizations have also been employed to incorporate thiolated affinity peptides that retain certain cell-survival proteins in the scaffold14, as well as proteins that mimic cell-cell interactions,15 thereby, integrating additional functional properties of the ECM.
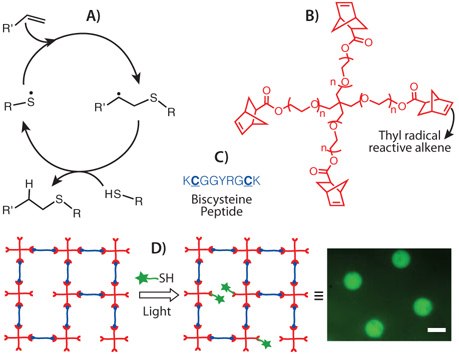
Figure 4.Thiol-ene photochemistry: A) General mechanism of the thiol-ene reaction; B) Structure of a commonly used ene, 4-arm PEG tetra norbornene; C) A cysteine containing chymotrypsin cleavable peptide crosslinker; D) Schematic of photopatterning networks formed via thiol-ene polymerization by reacting a network off-stoichiometry and subsequently conjugating a thiolated cell adhesive ligand at specific locations within the hydrogel scaffold; Image obtained from photopatterning of Alexafluor488 labeled RGD, scale bar: 500 μm.
Strain-promoted Azide-Alkyne Cycloaddition Reactions
Among various click reactions, copper (I) catalyzed azide-alkyne cycloaddition (CuAAC) to form 1,2,3-triazole (Figure 2A) has emerged as a widely utilized reaction for applications ranging from material to biomedical sciences, because azides, alkynes, and triazoles are largely inert to various reaction conditions and to most biological systems. While this click reaction gained popularity only after the introduction of a copper (I) catalyst into classical Huisgen cycloaddition, the toxic nature of this transition metal considerably restricted its utility in biological systems. Particularly for cell culture applications, CuAAC produced hydrogels simply remained as a prefabricated cell culture substrate rather than a cell encapsulation scaffold,16,17 while being the icebreaker for the emergence of sequential click reactions (see Sequential Click Reactions section) techniques in hydrogel scaffolds.18
To overcome this limitation of CuAAC, Bertozzi and co-workers pioneered the development of strain-promoted azide-alkyne cycloaddition (SPAAC) between cyclooctynes and azides (Figure 5) which proceeds without the need for copper catalyst, making it as an ideal bioorthogonal reaction suitable for cell encapsulation.9 Building from the concept of copper-free click chemistry, PEG hydrogels have been synthesized using 4-arm PEG tetraazide (Figure 6A) and di-cyclooctyneterminated matrix metalloproteinase (MMP) degradable peptides.19,20 Here, the gel is crosslinked using a degradable peptide to enable network degradation and remodeling, as dictated by cell-secreted MMPs. As one example, a gem-difluorocyclooctyne (DIFO)9,19 was employed for its faster kinetics due to the presence of electron withdrawing fluorines, along with the characteristic ring strain (Figure 5). Gelation occurs in less than 5 minutes under physiological conditions in the presence of cells.
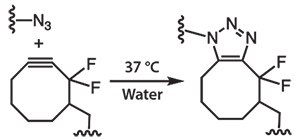
Figure 5.SPAAC between an azide and a difluorocyclooctyne eliminates the need for a copper catalyst and allows material fabrication under physiological conditions.
Sequential Click Reactions
In numerous biological applications, it is often desired to introduce functional cues at selected time points and/or in spatially defined regions. These signals may include survival cues (e.g., integrin-binding molecules), growth factors (e.g., affinity ligands to sequester cytokines), or cell-specific degradation sites (e.g., MMP-cleavable linkers to release signals or allow cell motility). This has led to the emergence of sequential click reactions: forming gels using one click chemistry and subsequently modifying it in a spatiotemporally controlled fashion using an additional click chemistry. Since thiol-ene chemistry is driven by light, it has become a powerful and versatile approach to achieve such well-defined and highly sophisticated incorporation of cues in PEG hydrogel cell niches.
Exploiting the orthogonality between SPAAC and thiol-ene click reactions: our research group has demonstrated the formation of PEG hydrogels via SPAAC (Figure 6A) followed by the creation of spatio and temporal photopatterns using the thiol-ene click reaction, in which case the peptide crosslinker carried a pendant allyl group (Figure 6A) to allow post-gelation photopatterning.19 Cells encapsulated within this hydrogel matrix not only expressed >90% cell viability, but also responded to the patterned biochemical cues. For example, spatial control over cell morphology and adhesions were established upon patterning RGD peptide at specific locations of the gel (Figure 6A). Similarly, when a selfquenching diflourescein collagenase cleavable peptide, which shows enhanced fluorescence upon cleavage, was photopatterned, much greater fluorescence was observed at regions of high collagenase activity i.e., around the cell and allowed to visualize the local activity of cells in real time.
More recently, we achieved sequential patterning and depatterning of biologically relevant cues using orthogonal photocoupling and photocleavage reactions (Figure 6B and 6C).21 Spatio and temporal control over coupling and cleavage were executed using light of different wavelengths using a thiolated RGD peptide that contained a nitrobenzyl ether linker21-23 to enable the photocleavage (Figure 6D) and subsequently allowed us to externally direct cell adhesion and detachment of human mesenchymal stem cell (hMSC) populations at specific locations within the hydrogel (Figure 6B and 6C). Such dynamic control over biochemical cues on hydrogel scaffolds enables one to not only spatially control release of specific signals and cell types for cell delivery applications in regenerative medicine, but also provide opportunities to tune stem cell differentiations by controlling the presentation of specific and multiple cues in a spatio-temporal manner.
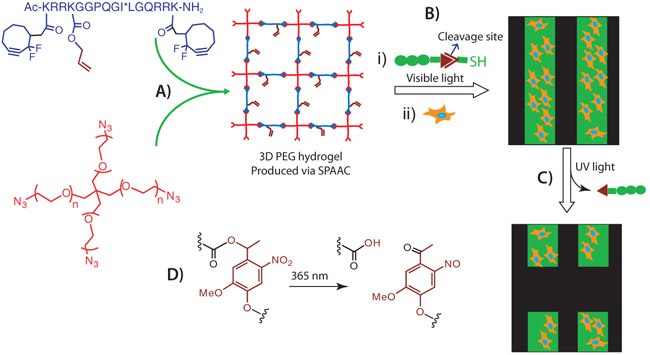
Figure 6.SPAAC Hydrogel Chemistry: A) 3D step-growth hydrogel formation via SPAAC click chemistry using 4-arm PEG tetra azide and dicyclooctyne MMP degradable peptide (* shows the cleavage site); B) Thiol-ene photopatterning on SPAAC produced hydrogel and subsequent cell adhesion; C) Spatial removal of patterned cues and resultant cell detachment; D) Nitrobenzyl ether photocleavage reaction.
Over the past decade, click chemistry has revolutionized the field of biomaterial scaffolds for cell culture and delivery because of its extreme convenience, versatility, and bio-orthogonal nature in building complex material scaffolds for regenerative medicine applications.24 This repertoire of reactions not only enables the creation of a highly functional class of PEG hydrogels, but also opens up innovative pathways, such as sequential click reactions to incorporate biochemical signals at specific locations of the hydrogel scaffold in a temporally controlled manner. However, many innovations in materials development are yet to emerge from this chemistry rich field, especially in the area of biomaterial scaffold design. For example, while these elegant chemical approaches have been greatly utilized to fundamentally understand and control cellular properties, including morphology and adhesion in a 3D environment, their utilization to better understand spatio-temporal complex cues in a stem cell niche and direct more efficient differentiation of stem cells in a controlled manner in vitro remains a great opportunity and challenge for biological engineers.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?