Click Chemistry Reagents Overview
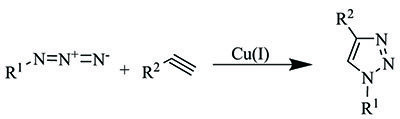
Our extensive portfolio of click chemistry reagents offers a variety of azides, alkynes, catalysts, and ligands to help accelerate your research in the exciting arena of “click” chemistry. Click chemistry is a term coined by Barry Sharpless to describe chemical reactions that are modular, efficient, wide in scope, provide very high yields, and generate only inoffensive byproducts. The most well-known example of a “click” reaction is the Copper(I)-catalyzed Azide-Alkyne 1,3-dipolar Cycloaddition (CuAAC), which yields a 1,4-disubstituted five-membered 1,2,3-triazole ring.
Products
Related Resources
- Introduction to Click Chemistry
Click chemistry is an approach to the synthesis of drug-like molecules that can accelerate the drug discovery process by using a few practical and reliable reactions.
- Copper-Free Click Chemistry
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
This reaction between azides and alkynes offers high yields and involves functionalities that can be introduced relatively easily in a variety of molecules such as synthetic polymers, fluorophores, small molecules or into specific locations in biomolecules. An advantage of this reaction for biological purposes is that the azide and alkyne functional groups are largely inert, or biorthogonal, towards biological molecules and aqueous environments. “Click” chemistry continues to gain popularity and is used in a variety of research fields with significant contributions to the fields of chemical biology, polymer chemistry, bioconjugation, and drug discovery.
Amino Acid Azides/Alkynes for Click Chemistry
Peptide synthesis using natural and non-natural amino acids is a powerful tool in the development of therapeutics and in understanding biological chemistry. We offer a variety of Fmoc- and Boc-protected azido amino acids for your peptide or amino acid-based chemical ligation needs, such as azide-alkyne cycloaddition reactions and Staudinger ligations.
Azide Sources for Click Chemistry
Incorporating azido functional groups into organic molecules is becoming an increasingly important task as these moieties continue to impact organic chemistry as well as biology in uses ranging from amino group protection to chemical ligation. We offer a wide selection of azide sources, from sodium azide to diphenyl phosphoryl azide, to facilitate azide synthesis and the preparation of tailor-made organic azides.
Organic Azides for Click Chemistry
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864, this energy-rich and versatile class of compounds has enjoyed considerable interest. Completely new perspectives have emerged, notably the use of organic azides for peptide synthesis, combinatorial synthesis, heterocycle synthesis, and the ligation or modification of biopolymers. The most prominent fields of application today are azide-alkyne cycloadditions and different variants of the Staudinger ligation. The azido group can also be used as a protecting group for primary amines, especially in sensitive substrates such as complex carbohydrates or peptide nucleic acids (PNA) and coordination compounds, as azides are stable to alkene metathesis conditions.
PEG Azides for Click Chemistry
PEG polymers contain numerous inherently favorable biological characteristics including high water solubility and a lack of toxicity and immunogenicity. Thus, the chemical modification of biologically active compounds, such as peptides, antibody fragments, enzymes, or small molecules with polyethylene glycol chains, referred to as “PEGylation”, often leads to improved pharmacokinetics and biological function in many applications. Our PEG azides are ideal starting materials for the synthesis of PEG derivatives via azide-alkyne cycloaddition or Staudinger ligation.
Trifunctional Probe Building Blocks for Click Chemistry
Small-molecule probes are widely used in chemical biology research for target ID/validation and the interrogation of biological systems. We have compiled a collection of trifunctional building blocks to facilitate the design and synthetic development of chemical probes. Each contains three components: a connectivity group, a reactive group, and a bio-orthogonal handle for downstream applications. Not only does the collection enable simultaneous incorporation of reactive groups, but the connectivity group (e.g. amine) can be leveraged to prepare libraries of probe analogs, allowing the biologist to screen for the optimal probe for a given assay.
Tetrazine/Strained-alkenes for Click Chemistry
The reaction of 1,2,4,5 tetrazines with strained alkenes has been applied as a rapid bio-orthogonal click chemistry reaction for biological labeling and cell detection applications among others. This reaction precedes rapidly via an inverse electron demand [4 + 2] Diels-Alder cycloaddition to yield a stable covalent linkage without the need for a catalyst and the only byproduct is dinitrogen. When using trans-cyclooctene, this reaction is orders of magnitude faster than azide-cyclooctyne-based click chemistry and has therefore found use in applications where low concentrations of reactants are desired or faster kinetics are needed.
Copper-Free Click Chemistry
Cu-free cycloadditions offer efficient ligation reactions useful for a variety of bioconjugation applications. When working with live cells these reactions offer the advantage of not having the cytotoxicity associated with Cu-catalyzed cycloadditions. We offer a suite of cyclooctynes, tetrazines and strained-alkene reagents for use in a variety of applications.
Staudinger Ligation
The reaction between an azide and a phosphine forming an aza-ylide was first reported in 1919 by Nobel Prize laureate Herrmann Staudinger. It has found widespread application in chemical synthesis and is valuable as a highly chemoselective ligation method for the preparation of bioconjugates. Both reactive functionalities involved in the Staudinger ligation reaction are bio-orthogonal and readily combine at room temperature in aqueous environments. These conditions make it possible to exploit the Staudinger ligation in complex cellular and organismal environments in the investigation of various processes in chemical biology. Our portfolio offers phosphine ligands for your various Staudinger ligation and conjugation applications.
To continue reading please sign in or create an account.
Don't Have An Account?