The Progress in Development of Dental Restorative Materials
Introduction
With dentists placing nearly 100 million dental fillings into patients′ teeth annually in the U.S. alone, polymeric composite restoratives account for a large share of the biomaterials market. These pigmented materials are composed mainly of liquid monomers and surface-functionalized particulate inorganic fillers, and can, upon polymerization, indistinguishably mimic the appearance of a natural tooth. While this natural appearance appeals to patients, the ability of the composites to bond to both dentin and enamel also makes polymeric composites a practical alternative to amalgam-based restorative materials. The matrix phase of currently used dental composites is predominantly based on dimethacrylate monomers. An example of a commonly used bulky aromatic dimethacrylate monomer is 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl]propane (BisGMA) (Prod. No. 494356)), which was originally developed by Bowen specifically for dental composite applications.1The bulky BisGMA monomer, which is extremely viscous due to strong intermolecular hydrogen bonding interactions, is normally used with lower viscosity comonomers such as urethane dimethacrylate (Prod. No. 436909), ethoxylated bisphenol A dimethacrylate (BisEMA, Prod. No. 455059) and triethylene glycol dimethacrylate (TEGDMA, Prod. No. 261548) among others.2 Efficient, rapid photopolymerization is typically achieved with visible light (wavelength 400–500 nm) using a suitable radical photoinitiator such as camphorquinone (Prod. No. 124893 along with a tertiary amine photoreductant such as ethyl dimethylaminobenzoate (Prod. No. E24905) or 2-(dimethylamino)ethyl methacrylate (Prod. No. 234907).3-5 Other initiating systems have also been reported, including bisacylphosphine oxides, titanocenes and germaniumbased compounds.6,7
Composite Restorative Materials
The glass-ceramic fillers in dental composites are typically aluminosilicate- based, with the inclusion of heavy metal oxides such as barium, strontium or zirconium to contribute X-ray opacity.8 The filler particle dimensions vary from approximately 0.1–10 μm for ground glass, to around 20–50 nm for pyrolytic or sol-gel derived silica and other nanomaterials. Organosilanes, mainly methacryloxypropyltrimethoxysilane (Prod. No. 440159), are used as coupling agents to provide the essential covalent connection between the filler and the polymer matrix. A combination of multiple filler particle sizes as well as silane surface treatment are used to incorporate a significant proportion (70–90 weight % or 30–55 volume %) of the high modulus reinforcing filler into the resin matrix (Figure 1). The high filler content in dental composites provides increased modulus, strength, abrasion resistance and toughness, as well as reduced thermal expansion. The filler component also limits the polymerization shrinkage associated with the transition of the liquid monomers to a highly cross-linked glassy polymer matrix. This in situ polymerization is necessary to achieve a restoration that is well adapted to the surrounding tooth structure.
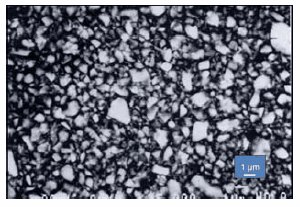
Figure 1.Scanning electron micrograph of a polished dental composite showing the irregularly sized inorganic particles embedded into a polymer matrix.
The intraoral placement restricts the photopolymerization conditions that can be employed to cure the restoratives, making the selection of materials significantly challenging. This unique placement has necessitated the development of ambient temperature, rapid, high conversion polymerization reactions that lead to high performance, aesthetic composite materials. The resulting restorative materials must be able to withstand substantial masticatory cyclic stresses (chewing stresses), as well as exposure to an aqueous environment involving temperature variations resulting from food and drink consumption. The critical adhesion of the composite to a tooth is reliant on an acid etch of dentin and enamel that demineralizes the surface and allows for strong micromechanical interlocking with a polymerizable adhesive layer.9 This layer serves as a transition from the relatively hydrophilic dentin within the tooth to the much more hydrophobic restorative composite.
Shrinkage and Stress in Restorative Composites
When bonded interfaces are imposed on a polymerizing sample of a composite, as is necessary with a dental restoration, the resultant restriction in free shrinkage induces significant internal and external stress (Figure 2a). This stress can cause a series of problems including: (a) deflection of the tooth, (b) failure of sections of the adhesive bond, and (c) defect formation within the polymer matrix at the filler-matrix interface, or in the adjacent enamel substrate.10
Reliable, long-term stability of the tooth-restoration interface has remained elusive, prompting substantial new materials research and development projects in both industrial and academic laboratories. The concerns with polymerization shrinkage and stress are not unique to dental polymers, and the research can also be applied to other industrial polymer applications including coatings, adhesives, encapsulants, aspheric lenses and photolithography. Stress is defined as a product of strain and modulus (σ = ε × Ε), where σ is the stress, ε is the strain, and Ε is Young′s Modulus. Stress development during the formation of glassy polymers in a polymerizing composite is a major concern. Therefore a basic understanding of the evolution of shrinkage strain and elastic modulus is critical to begin solving the stress/strain problems. The values of the shrinkage strain and modulus are dependent on the degree polymerization, along with temperature change in the non-isothermal composite photopolymerizations. Dimethacrylate monomers are capable of forming polymer networks with glass transition temperatures above the curing temperature, but this usually means that a significant percentage of the methacrylate functionality remains un-reacted in the fully cured polymer.11
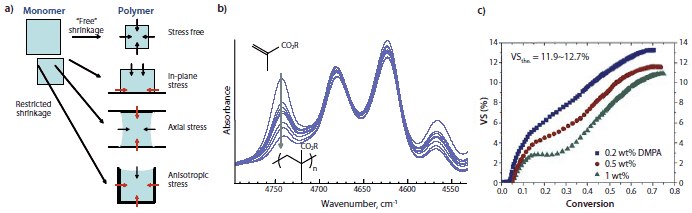
Figure 2. (a).Restricted shrinkage associated with bonded surfaces during polymerization leads to significant stress development within a polymerizing sample. (b) Near-infrared spectroscopy focused on the methacrylate =CH2 combination band can be used to monitor reaction kinetics and conversion of BisGMA/TEGDMA monomers during polymerization. The grey arrow at 4745 cm-1 indicates the consumption of monomer, showing the progress of the polymerization reaction. (c) Non-linear dynamic volumetric polymerization shrinkage (VS) of TEGDMA photoinitiated with various concentrations of 2,2-dimethoxy-2-phenylacetophenone (DMPA, Prod. No. 196118) as a function of conversion, demonstrating thermal expansion/contraction effects and delayed shrinkage in the glassy state. From the graph, the glassy state begins at approximately 0.45 conversion of monomer.
During the photopolymerization, a liquid monomer converts to a crosslinked polymer by passing through the stages of gelation, rubbery regime and vitrification (transition into a glassy state) as a function of the monomer to polymer conversion progress. A series of hybrid analytical techniques have been developed that employ real-time near-infrared spectroscopy to monitor the polymerization reaction rate and conversion simultaneously with dynamic measurements of volumetric shrinkage, modulus or stress during the photopolymerization process (Figure 2b).10,12 These studies have demonstrated that shrinkage is nonlinear with respect to conversion due to both thermal excursions during polymerization and a reduced rate of shrinkage relative to conversion in the glassy polymeric state. Modulus and stress development were found to be concentrated in the later stages of conversion and at the onset of vitrification (Figure 2c). Based on this information, and on the assumption that stress reduction should not be accomplished by limiting either conversion or modulus of the polymer, there are several modern approaches with practical potential to produce lower stress dental polymers.
Designing Bulky Monomers
One possible solution to the polymerization shrinkage problem is the development of new monomers and reactive oligomers that are customized for dental composite applications. A decrease in the initial reactive group concentration generally results in a reduced modulus, but also results in a lower crosslinking density within the polymer network. This unintended consequence may be avoided, for example, by designing a large monomer with a lower initial reactive group concentration, but with an auxiliary reinforcement mechanism to counter the lower covalent crosslink density.
Materials based on the relatively large BisGMA monomer demonstrate anomalously high mechanical strength despite their relatively low limiting conversion and crosslinking density values, most likely as a consequence of hydrogen bonding between the hydoxy and carbonyl groups. This larger monomer strategy, combining physical and covalent crosslinking, is also used in significantly higher molecular weight dimethacrylate monomer analogs of BisGMA, such as the di-tert butyl phenol and methacrylic acid substituted monomers synthesized from bisphenol A diglycidyl ether (Figure 3a).13 In the case of the bis(di-tert butylphenoxy)- modified dimethacrylate (DtBP-BisGMA), the steric interactions of the added bulky aromatic groups provide additional physical network reinforcement, allowing low shrinkage and high modulus to be collaboratively maintained.
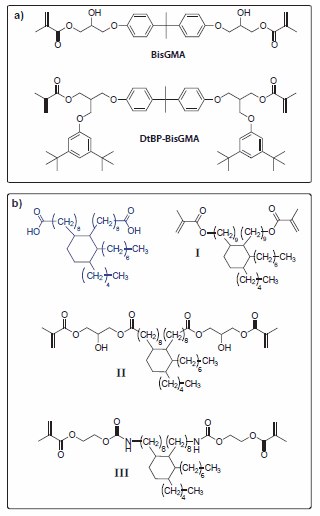
Figure 3. (a)BisGMA (MW = 513) is a representative dimethacrylate monomer commonly used as a co-monomer to form the highly cross-linked polymeric matrix phase in dental composites. The related DtBP-BisGMA (MW = 899) bulkier monomer structure provides polymers with lower polymerization shrinkage and reduced covalent crosslink densities, while maintaining good strength due to physical network reinforcement by the bulky substituent groups. (b) Dimethacrylate monomers based on the C36 diacid core structure (blue). Monomer I was prepared by reduction of the diacid to the diol followed by a reaction with methacrylic anhydride. Monomer II was synthesized using a diepoxide analog of the core structure and reacting it with methacrylic acid, while monomer III was synthesized by the reaction of a diisocyanate analog with 2-hydroxyethyl methacrylate (Product No. 477028). As co-monomers with selected conventional dimethacrylates, these monomers lead to polymerizationinduced phase separation that contributes to lowering of the shrinkage, translating into a reduction in stress.
Copolymer Development
In a different application of bulky monomers to lower polymerization shrinkage, a series of dimethacrylate compounds were prepared from a C36 diacid core structure, which results in completely amorphous cured polymers (Figure 3b). Beginning with the C36 diacid or the corresponding diepoxide, several monomers have been prepared with different connecting groups between the terminal methacrylate groups and the C36 core.14 All these materials produce homopolymers that exhibited high conversions, along with low shrinkage and extreme hydrophobicity, while remaining rubbery and low modulus at room temperature. When combined as co-monomers with other conventional dental monomers, it was noted that C36 diacid monomers lack the ability to hydrogen bond, and showed limited affinity for hydrogen bonding with monomers such as BisGMA. Conversely, the introduction of a hydrogen bonding -OH group into the C36 diacid monomer structure imparted compatibility with BisGMA, but not with the ethoxy analog, BisEMA. This limited thermodynamic compatibility can be adjusted based on the binary or ternary co-monomer compositions such that initially homogeneous monomer mixtures produce copolymers with controlled degrees of heterogeneity.
Despite high conversion in the final heterogenous polymers, exceptionally low polymerization shrinkage can be obtained under specific conditions of relative reaction kinetics within the separate phases, and the overall phase structure in the polymerized copolymer material. These heterogeneous copoloymer materials demonstrate the potential for late-stage shrinkage recovery, and thus stress relaxation, due to the concentration of stress development toward the end of the polymerization reaction. Thermoplastic prepolymer additives, so-called low-profile additives, have also been shown to control shrinkage based on internal stress relief at phase boundaries created during the polymerization-induced phase separation.
Preparation of Nanogels
Highly branched, short-chain nanogels have been prepared from relatively concentrated monovinyl/divinyl polymerizations involving solutions of isobornyl methacrylate (Prod. No. 392111) and urethane dimethacrylate (Figure 4).15 The discrete globular polymeric nanogel particles have sizes between 5 and 100 nm with molecular weights of 104 to more than 106 Da. These methacrylate functionalized nanogel particles can be added to dental resins at concentrations up to 50 wt. %, with at least proportional reduction in polymerization shrinkage and stress development. There is also little to no effect on the curing reaction kinetics, conversion, and mechanical strength properties. At levels up to about 20 wt. % of the nanogel, there is only a limited effect on monomer viscosity. Even at high nanogel loading levels, high amounts of inorganic filler can still be incorporated to give low shrinkage, low stress dental composite materials.
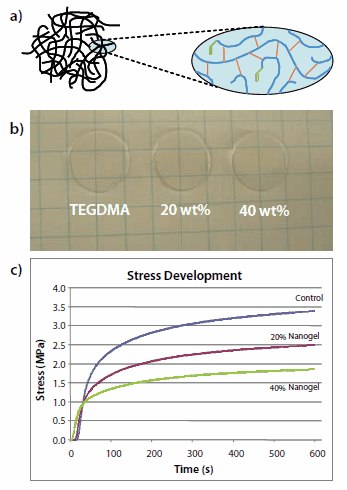
Figure 4. (a). Branched/cyclized nanogel particles can be made from solution copolymerization of monovinyl and divinyl monomers (2:1 molar ratio) with a chain transfer agent used to control chain length, avoid macrogelation, and provide a site to reintroduce reactive methacrylate groups. (b) Even at high concentrations, reactive nanogel particles dispersed in TEGDMA (Prod. No. 261548) provide nearoptically transparent monomeric and polymeric materials. (c) The polymerization shrinkage and stress are significantly reduced, without detrimental reduction of monomer conversion or mechanical properties in the final nanogel-modified polymer.
Methacrylate Cross-Linked Networks Using Thiol-ene Chemistry
There are also methods to reduce polymerization stress that are relatively independent of the shrinkage generated during the curing process. Rather than relying exclusively on free radical chain growth polymerization of methacrylates, a radical-induced thiol-ene polymerization involves a step growth mechanism. Molecules with multiple thiol groups, such as pentaerythritol tetramercaptoproprionate (Prod. No. 381462), can be photoinitiated and reacted with compounds containing multiple alkene groups, such as triallyl-1,3,5-triazine-2,4,6- trione (Prod. No. 114235), to yield extremely high conversions and highly homogeneous polymer networks.16,17 Another advantage is that these thiol-ene polymerizations provide well-controlled gel points, which occur at much higher conversions than those of dimethacrylate polymerizations. This is important since shrinkage prior to gelation is accommodated by viscous flow with virtually no stress development within the sample. Therefore, thiol-ene polymerizations with gel points between 40 and >70% conversion are possible based on the step growth reaction process, translating into polymers with dramatically reduced final stress levels compared with polymers made by dimethacrylate polymerization.
In a thiol-ene polymerization, the alkene is typically selected to eliminate or minimize homopolymerization of the -ene component. In this regard, alkenes based on vinyl ethers, vinyl esters, allyl ethers and norbornene functional groups are well suited to maintain a stoichiometric consumption rate with the thiol groups. Mixed thiol-ene/methacrylate resin systems that polymerize through a hybrid step/chain-growth mechanism provide advantages in terms of improved shelf-life stability and enhanced mechanical strength, while still resulting in low stress polymers.18 On the one hand, as explained above, thiol-alkene reactions are able to generate step-growth networks with high crosslink densities. On the other hand, thiols are well known to function as effective chain transfer agents with methacrylate monomers. In a simple thiol-modified dimethacrylate photopolymerization, methyl mercaptopropionate (Prod. No. 108987) and benzenethiol (Prod. No. 240249) demonstrate well-controlled delayed gelation and vitrification leading to a higher final polymeric conversion and modulus, but with significantly reduced stress.18
A completely different approach to alleviate stress in polymer networks was recently demonstrated for thiol-ene systems, and is also applicable to crosslinked dimethacrylate materials. This novel approach involves creation of a covalent adaptable network in which the bond structure of the network remains covalent, yet each individual bond can be broken and reformed in the presence of active radical species.19 In a dental materials application, this approach can be achieved by incorporating allyl sulfide moieties into multifunctional monomers that subsequently undergo a thiol-ene photopolymerization. As polymerization proceeds, addition-fragmentation of the allyl sulfide linkages allows the developing polymer network to relax stress throughout the polymerization rather than simply before gelation (Figure 5). This adaptive network process has been shown to result in polymerization shrinkage stress reduction of up to 75% when compared with the otherwise identical system of crosslinking monomers with the allyl sulfide group replaced by a propyl sulfide analog.

Figure 5.Covalent adaptable network structure based on crosslink units with allyl sulfide groups capable of radical addition-fragmentation to relieve stress during the polymerization. The presence of additional thiol terminated polymer chains allows for the reversible random replacement of existing carbon-sulfur linkages leading to the dissipation of stress throughout the cross-linked polymer network.
Conclusions
Composite materials based on methacrylate monomers are commonly used to provide aesthetic and functional restoration of dental tissue. A broad spectrum of polymerization chemistries is being developed to further improve the reliability of these materials. Approaches under investigation include the design of new monomers and the use of novel polymerization mechanisms. These approaches are directed toward addressing the ongoing challenges associated with shrinkage during polymerization and stress within the restorative composites, but can also be applied to other industrial polymers.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?