Syntheses of Functionalized Alkenes, Arenes, and Cycloalkenes via a Hydroboration-Coupling Sequence
Abstract
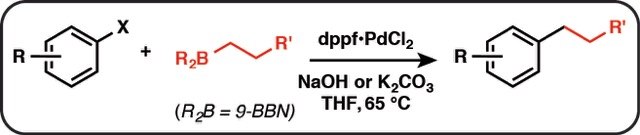
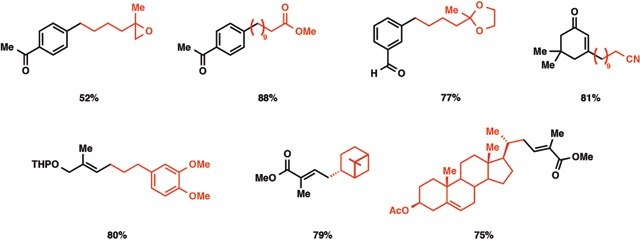
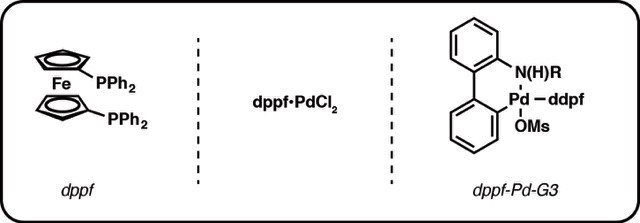
The cross-coupling reaction of B-alkyl-9-borabicyclo[3.3.l]nonanes (B-R-9 BBN), readily obtainable from alkenes by hydroboration, with 1-halo-1-alkenes or haloarenes in the presence of a catalytic amount of PdCl2(dppf) and bases, such as sodium hydroxide, potassium carbonate, and phosphate, gave the corresponding alkenes or arenes. Because the reaction is tolerant of a variety of functionalities on either coupling partner, stereochemically pure functionalized alkenes and arenes can be obtained under mild conditions. The utility of the reaction was demonstrated by the stereoselective synthesis of 1,5-alkadienes and the extension of a side chain in a steroids. The hydroboration of haloalkadienes, followed by the intramolecular cross-coupling, gave a short-step procedure for synthesis of cycloalkenes, benzo-fused cycloalkenes, and exocyclic alkenes.
Procedure
A dry 50-mL flask equipped with a magnetic stirring bar, a septum inlet, an oil bubbler, and a reflux condenser was flushed with nitrogen. To the flask were added an alkene (5.5 mmol) and dry THF (2.5 mL) and then a solution of 9-BBN (0.5 M solution in THF, 5.5 mmol) at 0 °C. The mixture was warmed up slowly to room temperature and then stirred for 4-6 h to give a solution of B-alkyl-9-BBN. Procedure A. To the above solution were added PdCl2�(dppf) (0.15 mmol), haloarene or 1-halo-1-alkene (5 mmoI), additional THF (12 mL), and aqueous NaOH (5 mL of 3 M solution) at room temperature. The mixture was refluxed overnight (ca. 14-16 h). After the reaction was completed, the reaction mixture was diluted with hexane or benzene (20 mL), and the residual borane was oxidized by addition of H2O2 (30%, 2 mL) at room temperature. The product was extracted, washed with brine, and dried over MgSO4,. When a product was sensitive to the alkaline hydrogen peroxide oxidation, the extracts were directly subjected to column chromatography without oxidation.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?