Heparin Versus Heparin Sulfate Proteoglycans
- Brief Summary
- Nomenclature
- Heparin vs. Heparan Sulfate
- Differences between heparin and heparan sulfate
- Chemical Structure
- Synthesis and Chemical Diversity
- Heparan Sulfate Proteoglycans
- Binding Partners
Brief Summary
Heparan sulfate (HS) is a component of the extracellular matrix. It is a glycosaminoglycan that is covalently attached to core proteins to form proteoglycans. Its structure is amazingly diverse since during synthesis it undergoes extensive sulfation and epimerization. Heparin is distinct from HS in that it is produced primarily by mast cells, whereas, HS is produced by all cell types. Note that there is not a single HS or heparin structure. Instead, the basic polysaccharide components that make up these molecules vary depending upon the tissue and the developmental stage.
Nomenclature
Heparin ≠ Heparan sulfate
Heparan sulfate = HS
Heparan sulfate proteoglycan = HSPG
Heparin vs. Heparan Sulfate
Heparin is only produced by mast cells. It functions as an anticoagulant. HS is made by almost all cell types and also has anticoagulant activity, but it is much lower than that of heparin. HS further varies from heparin in the degree of modification of the sugar residues. The table below summarizes the differences between the two molecules.
Differences between heparin and heparan sulfate
Chemical Structure
Heparin and HS are glycosaminoglycans (GAGs), which are linear polysaccharides composed of two basic saccharides: an amino sugar and an uronic acid. The amino sugar is typically either N-acetyl-D-glucosamine (D-GlcNAc) or N-acetyl-D-galactosamine (D-GalNAc). The uronic acid is either D-glucuronic acid (D-GlcA) or L-iduronic acid (L-IdoA). These basic components are further varied by epimerization, sulfation, and deacetylation. Elongation and modification of the polysaccharides are thought to occur simultaneously during biosynthesis. Heparin and HS are based on a polysaccharide unit of:
- GlcNAc (α1-4)
- GlcA (β1-4)
- GlcNAc (α1-4)
- GlcA (β1-4)
- GlcNAc-N-sulfate-6-sulfate α1-4
- GlcA (β1-4)
- GlcNAc-N-sulfate-6-sulfate α1-4
- IdoA-2-sulfate (α1-4)
- GlcNAc-N-sulfate (α1-4)
- GlcA (β1-4)
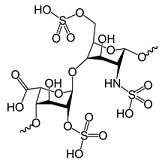
Where:
GlcNAc = N-acetylglucosamine
lcA = Glucuronic acid
IdoA = Iduronic acid
They are typically covalently attached to proteins to form proteoglycans.
Synthesis and chemical diversity
The heparin disaccharide is synthesized by α-GlcNAc Transferase II and β-GlcA Transferase II. The sugar moities are then modified by at least four sulfotransferases (N-Sulfotransferase, 2-O-Sulfotransferase, 3-O-Sulfotransferase and 6-O-Sulfotransferase) and one epimerase, GlcA-C5-Epimerase. 3’-phosphoadenyl-5’-phosphosulfate (PAPS) donates the high energy sulfate groups that are covalently attached to the HS. These enzymes do not consistently modify the HS chain. Instead they act through regions so that the resulting HS polymers have areas with high degrees of sulfation and other areas with none. This results in HS chains with a staggering degree of heterogeneity. Furthermore, the patterns of epimerization and sulfation set the ligand binding sites thus changing the functionality of the chains.
Heparan Sulfate Proteoglycans
A proteoglycan (PG) is composed of a core protein with one or more covalently attached GAGs. PGs are stored in secretory granules, inserted into the plasma membrane or secreted into the ECM. The table below lists several well characterized HS proteoglycans (HSPG) along with their molecular weight and tissue localization.
Binding partners
Heparin/HS have several well characterized binding proteins. Of these, one of the most extensively studied is the interaction between heparin and FGF2 (bFGF). A crystal structure of this interaction has been solved. The heparan sulfate proteoglycan (HSPG) perlecan sequesters and regulates the release of FGF, VEGF and other cytokines involved in neovascularization.
Materials
Reference
如要继续阅读,请登录或创建帐户。
暂无帐户?