用于细胞追踪的亲脂性染料试剂盒
1 改编自:Tario Jr.等人 (2011) 的表1;经Humana出版社许可转载。最初发表于《Mol. Biology》, 699, 119 (2011).
缩写:APC = 别藻蓝蛋白; 7-AAD = 7-氨基放线菌素D; CFSE = 羧基荧光素琥珀酰亚胺酯; Cy3 = 花青3; Cy5 = 花青5; Cy7 = 花青7; FP = 荧光蛋白; PE = 藻红蛋白; PI =碘化丙锭; PerCP = 多甲藻素叶绿素 l 蛋白; TR =Texas Red(德克萨斯红)*
* CellVue是PTI Research, Inc.的商标。CY是Cytiva的商标。Texas Red和TO-PRO-3是Molecular Probes的商标。DRAQ5是Biostatus Ltd.的商标。
1 详情参见参考文献1-4和11。PKH26最初是在1980年代后期由Paul Karl Horan及其同事在Zynaxis Cell Science开发的,我们从1993年和1997年便开始销售PKH26和PKH67。CellVue Claret,一种由PTI Research, Inc.开发的远红类似物,于2008年加入了我们的细胞追踪家族。
2 光谱的兼容和不兼容是具有代表性而非穷举性的,它们假定激光激发点在空间上是分开而非一致的。来自不同荧光探针的相对信号随目标生物系统的不同而不同。每个用户必须运行必要的对照来检验在其自己系统中的兼容性。
3 Shcherbo D, Merzylak EM, Chepurnykh TV et al. Bright Far-red Fluorescent Protein for Whole-body Imaging.Nature Meth.4:741-746 (2007).
4 当FBS或其他蛋白质在染色方案中用作“终止”试剂时,24小时时 < 0.3%(见文本)。
5 对于PKH染料应用的部分参考书目(1988-2006),见表1和表2。
6 详情参见参考文献3、8、9、12和20。
7 详情参见参考文献3和11。
8 GL试剂盒含有0.5 mL染料+ 6x10 mL稀释剂C(推荐用于大规模或体内研究中细胞的一般膜标记)。
9 MIDI试剂盒含有2x0.1 mL染料+ 6x10mL稀释剂C(推荐用于体外增殖或细胞毒性研究中细胞的一般膜标记)。
10 MINI试剂盒含有0.1 mL染料 + 1x10 mL稀释剂C(推荐用于小规模或初步体外研究中细胞的一般膜标记)。
11 PCL试剂盒含有0.5 mL染料 + 6x10 mL稀释剂B(推荐用于体外或体内研究中,在非吞噬细胞存在下,选择性标记吞噬细胞)。
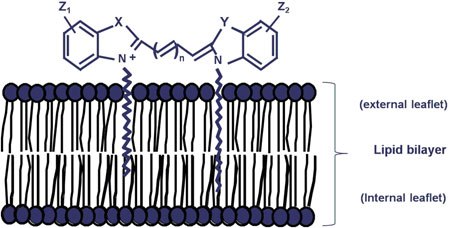
图S1.染色机制
PKH和CellVue染料是具有荧光“头部基团”和长脂肪族“拖尾”的脂质样分子(未按比例显示)。在含盐缓冲液或培养基中,它们迅速形成胶束或聚集体,对于非吞噬细胞具有较差的细胞标记效率。稀释剂C(是我们的通用膜标记试剂盒中所含的等渗无盐、无溶剂染色载体)使染料溶解度最大化,并促进近乎瞬时分配到脂质双层中。与周围脂质拖尾的强有力的非共价相互作用,对非分裂细胞具有长期染料保留能力和稳定的荧光强度。
经John Wiley & Sons, Inc.许可转载。首次发表于Cytometry, 73A.1019 (2008).
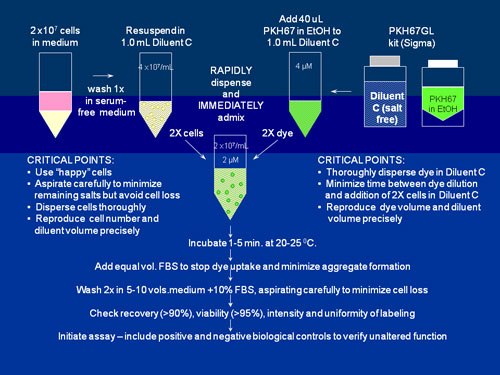
图S2.PKH26、PKH67和CellVue Claret的一般膜标记方案
当使用我们的细胞接头试剂盒中所含的无盐稀释剂C载体以最大化染色效率和均一性时,这些高度亲脂性染料在与细胞混合时几乎立即分配到细胞膜中。如该示意图中用PKH67标记的情况,通过以下方法能最容易获得明亮、均匀和可再现的染色:1)最大限度地减少染色步骤中的含盐量,以及2)确保细胞在染料中的快速均匀分散。(如需详细的方法和染色试验方案,可参见参考文献2和3以及表S2中包含的单个产品公告链接。)

图S3.靶群体的色码使得能够同时比较体内CTL细胞毒性与单个小鼠中的多个表位
未加标的脾细胞,或者用4种免疫原性MHV-68肽中的1种在37℃下加标1小时后的脾细胞,用3种不同的细胞追踪染料中不同的1色或2色组合进行标记。用CMTMR单染色鉴定未加标的脾细胞,而加标肽的脾细胞用CellVue Claret和CFSE的组合标记。然后将5个标记的靶标群体以相同的数量混合,注射到健康小鼠以及10天前感染了MHV-68病毒的小鼠(每组3只),在注射4小时后收获脾脏。在红细胞裂解后,用流式细胞仪评估每个群体中存活靶标的频率,使用光散射选通排除碎片和聚集物以及7-AAD摄取以排除无活性细胞。[注意:尽管此处显示的肽是抗病毒疫苗的候选表位,但类似的策略可用于鉴定有效的抗肿瘤疫苗成分。]
A. 代表性的两色图,显示用于鉴定用每种肽加标的靶群体的追踪染料组合(仅用CMTMR:无肽;CFSEhi CellVue Claretneg:ORF61p;CFSElo CellVue Claretneg:ORF6p;CFSEneg CellVue Claretpos:ORF9p;CFSEhi CellVue Claretpos: B8Rp)。
B. 代表性单色直方图,用于确定消灭的表位特异性。左图:CMTMR选通的。中图:CellVue Claretneg靶标的CFSE分布。右图:CellVue Claretpos靶标的CFSE分布)。下图中的值表示特异性裂解百分比,基于在感染动物与未感染动物中观察到的成活靶标的频率变化计算而得(详见参考文献9)。
经Informa Healthcare许可重印.首先发表于Immunol. Invest.36, 829 (2007)。
产品列表
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?