Separations of Tetracycline Antibiotics by Reversed Phase HPLC, Using Discovery Columns
There are more than 100 antibiotics on the market today, and many more are in development. Resistance to antibiotics is a significant problem (1,2): an antibiotic that takes a decade to bring to market can induce resistance within months of its introduction into clinical practice (3). The frequency of resistance in bacteria and the numbers of drugs to which they are resistant are increasing. Therefore, it is critical to monitor the level of antibiotics given to humans and animals.
HPLC is a powerful tool for isolation and quantification of antibiotics. In this application, five tetracycline antibiotics (Figure A) were analyzed by HPLC, using Discovery C18, Discovery C8, and Discovery RP-AmideC16 columns. Tetracycline antibiotics have a broad spectrum of activity, are relatively safe, and are effective against many infections caused by Gram-negative and Gram-positive bacteria (4,5).
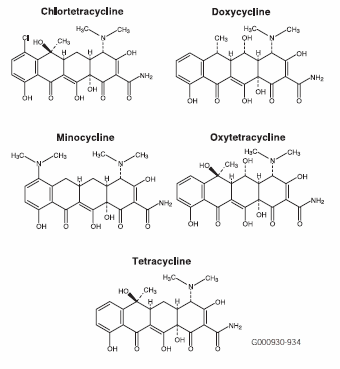
Figure A.Structures of Tetracycline Antibiotics
Chromatographic separations were performed on a Waters Alliance HPLC system. All injections were made through an autosampler. A Waters 2487 dual wavelength UV detector was used to monitor the UV absorbance of samples at 260nm. The 15cm x 4.6mm ID Discovery C18, Discovery C8, and Discovery RP-AmideC16 reversed phase HPLC columns were used without guard columns or filters. The packing particles in all columns were 5μm in diameter.
Doxycycline, minocycline, tetracycline, chlortetracycline, and oxytetracyline were dissolved in 25mM KH2PO4 buffer, pH 3.
The five tetracycline antibiotics were separated by gradient elution. Column temperature was controlled at 35°C. Column pressure was below 1050psi in all cases. Detailed conditions for each analysis are presented with the corresponding chromatogram.
Separations of the five tetracyclines are illustrated in Figures B, C, and D. Minocycline and oxytetracycline will coelute from the C18 column, but are very well separated by the RP-AmideC16 and C8 columns (Figures C and D). The Discovery RP-AmideC16 column provides the best resolution of minocycline and oxytetracycline. The background around the chlortetracycline and doxycycline peaks is caused by impurities in the samples. Note that on-column quantities of analytes differed among Figures B, C, and D.
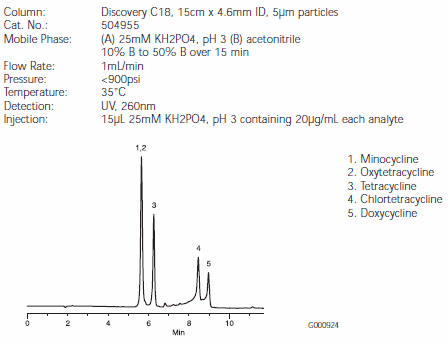
Figure B.Tetracycline Antibiotics on a Discovery C18 HPLC Column (504955)
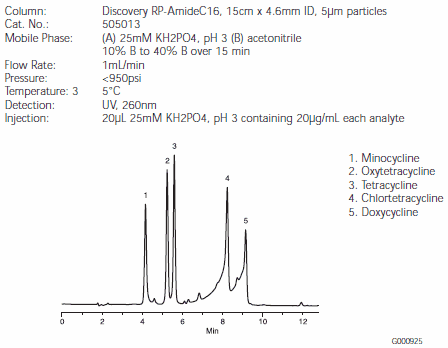
Figure C.Tetracycline Antibiotics on a Discovery RP-AmideC16 HPLC Column (505013)
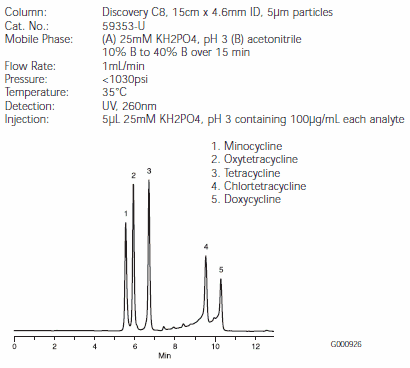
Figure D.Tetracycline Antibiotics on a Discovery C8 HPLC Column (59353-U)
The ability of the RP-AmideC16 phase to separate minocycline and oxytetracycline might be explained by the hydrogen bonding between the amide functionality of the phase and the hydroxy functionality of oxytetracycline. Such differences in selectivity show the advantage of using the amide column for a difficult separation.
This study showed that mixtures of tetracycline antibiotics could be separated by reversed phase HPLC, using Discovery C18, Discovery C8, and Discovery RP-AmideC16 columns. Except for coelution of the tetracyclines minocycline and oxytetracycline on the Discovery C18 column, excellent resolution was achieved in every separation.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?