Analysis of Active Cannabis Compounds
Introduction
Potency testing in marijuana-infused edibles is a problematic task that analytical labs are facing due to the complexity of the involved matrices. Among the most popular matrices are gummy bear candies and brownies. According to one laboratory site, the concentration of active ingredients in the edibles can range from a few parts per million to 3.5 parts per thousand.1 In this application, a procedure was developed to extract active cannabinoid compounds from gummy bears and brownies. The procedure included a simple and fast extraction of the active compounds from the studied foods, and analysis by HPLC-UV using a biphenyl stationary phase chemistry.
Experimental
Cerilliant® cannabinoid standards, available as 1 mg/mL solutions in either methanol or acetonitrile, were used for this experiment. The concentration of cannabinoids allowed for the spiking of both gummy extract and brownies at about 40 ppm with all compounds. The following compounds were included in this study: cannabidivarinic acid (CBDVA), cannabidivarin (CBDV), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabidiolic acid (CBDA), cannabidiol (CBD), tetrahydrocannabivarin (THCV), cannabinol (CBN), (-)-Δ9-Tetrahydrocannabinol (Δ9-THC), (-)-Δ8- Tetrahydrocannabinol (Δ8-THC), and (-)-Δ9-Tetrahydrocannabinolic acid A (THCAA). This list of 11 different cannabinoids includes several acidic forms; thus HPLC analysis was used in order to quantitate these in their native forms.
The HPLC column used was Ascentis® Express Biphenyl, 2.7 µm particle size, which gave the best separation of all 11 compounds in under 13 minutes. The use of this column with Fused-Core® particle architecture resulted in low back pressure, thus a standard pressure HPLC system could be used during this experiment.
Sample Preparation
One gummy bear candy, non-spiked, (2.3 g) was dissolved in 20 mL of warm water. This solution was then spiked with cannabinoids and extracted using a QuEChERS procedure. The average spiking level in each gummy bear was 45 ppm for each compound. Bears of four different colors were tested – orange, yellow, red, and green. After spiking, the water/candy solution was transferred to a 50 mL plastic QuEChERS extraction tube (55248-U). Acetonitrile (10 mL) was added, and the tube was shaken for one minute by hand. Supel™ QuE non-buffered salts (55295-U) were added, and the samples were shaken for 5 minutes on an automated QuEChERS shaker. Post-shaking, the samples were centrifuged for 5 minutes at 5000 rpm. The top layer was collected and injected directly into the HPLC.

For brownies, a 2.5 g sample of a non-spiked brownie with frosting was added to the QuEChERS extraction tube. This sample was spiked with cannabinoids and allowed to sit for 30 minutes prior to extraction. The average spiking level for the brownies was 40 ppm. The QuEChERS extraction was performed as previously described for gummy bears. Post-extraction, the top acetonitrile layer was collected into a vial and kept under refrigeration for a minimum of 3 hours to remove fats prior to HPLC analysis.
A calibration curve was constructed in acetonitrile bracketing the expected concentration of 10 µg/mL in the final extracts. The following calibration points were included: 2 µg/mL, 5 µg/mL, 10 µg/mL, 20 µg/mL and 25 µg/mL.
Results and Discussion
For the gummy bear samples, it was found that neither the red, yellow, nor green color interfered with detection of cannabinoids at 220 nm. The red color was partially extracted into acetonitrile, while the green and yellow colors stayed in the aqueous layer upon extraction. However, the orange color from the gummy bear, when extracted into acetonitrile, was found to have an interfering peak that co-eluted with CBDVA. Thus, for the orange gummy bear, quantitation of CBDVA was done at 280 nm, where CBDVA has significant absorbance free of interference. Quantitation was done at 220 nm for the rest of compounds in this study (Figure 1).
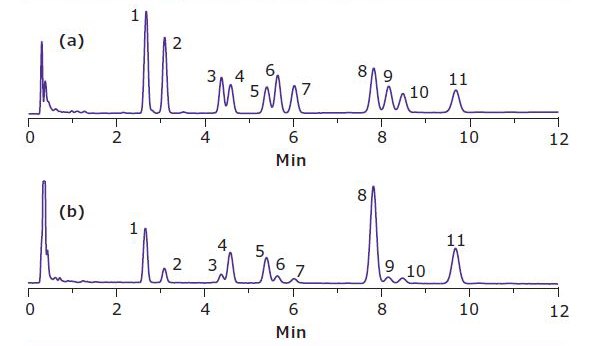
Figure 1. HPLC Chromatogram of Orange Gummy Bear Extract at (a) 220 nm and (b) 280 nm Figure 2. HPLC of a Brownie Extract at 220 nm. The peak elution order is listed in Table 1.
While no cleanup was required for gummy bear samples post-extraction, the co-extractives in the brownie were found to decrease the recoveries of the analytes if the brownie extract was injected into HPLC without further processing. The brownie extract was cleaned by refrigeration to remove the co-extracted fats.
The ruggedness of the method for brownies was tested by injecting the brownie extract (Figure 2) multiple times followed by the injection of the 10 µg/mL standard. After 7 injections of the brownie extract, it was found that the peak retention times were not affected, indicating that the column was being thoroughly cleaned between injections. The peak areas for the standards showed a slight decrease of 4 %.
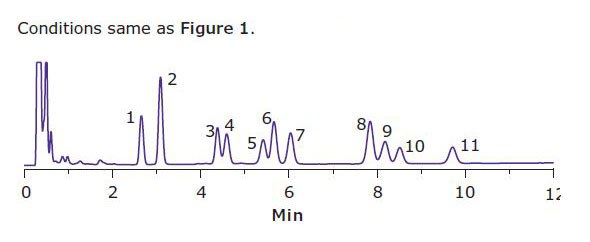
Figure 2.HPLC of a Brownie Extract at 220 nm The peak elution order is listed in Table 1.
Excellent recovery values of above 90 % for gummies and above 80 % for brownies were achieved with good accuracies (Table 1).
*The orange gummy was done at 280 nm due to the interfering background peak quantitation.
Note: THCA is the abbreviation used by AOAC
Conclusion
A method was developed for analysis of active cannabinoid compounds in both brownies and gummy bears. The extraction procedure involved a salting out step into acetonitrile and did not require intensive cleanup. The separation of eleven compounds was achieved on a biphenyl stationary HPLC phase and was completed in 13 minutes. The active compound CRMs are available from Cerilliant® through SigmaAldrich.com.
Materials
Reference
To continue reading please sign in or create an account.
Don't Have An Account?