LC-MS/MS Analysis of 16 Per- and Polyfluoroalkyl Substances (PFAS) in Milk Using QuEChERS Based on FDA Method C-010.02
Section Overview
Introduction
Per- and polyfluorinated alkyl substances (PFAS) are a class of compounds that have been widely used in commercial product applications over the past decades due to their versatile physical and chemical properties (e.g., water repellent, firefighting foams, cookware, food packaging). Owing to their chemical stability, these compounds are also widely present in our environment and have the potential to bioaccumulate in humans over time. Regulatory agencies such as the EPA and FDA have introduced limit values for certain substances and the development of analytical methods for PFAS testing to avoid possible human health risks (such as low infant birth weights, cancer, and effects on the immune system).1-4
The U.S. Food and Drug Administration (FDA) has issued a methodology (C-010.02) for PFAS extraction from food samples applying a modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) extraction technique and further clean-up step using dispersive solid phase extraction (dSPE), followed by LC-MS/MS analysis.4
For FDA method C-010.02, an extraction salt mixture containing 6.0 g MgSO4 as well as 1.5 g NaCl, and a dSPE clean-up mix containing 900 mg MgSO4, 300 mg PSA and 150 mg graphitized carbon, are specified. The Supel™ QuE non-buffered extraction salt mix and the specifically designed Supel™ QuE PSA/ENVI-Carb™ Tube 3 for clean-up have been used to meet the method requirements.
This application note describes the analysis of 16 PFAS compounds in milk and was performed in accordance with FDA method C-010.02.
Experimental
Solutions and Standards Preparation
Native and isotopically labeled PFAS standards were used as methanolic 50 µg/mL stock solutions. These standards were then diluted following the dilution scheme of the method C-010.02 to obtain calibration standards in the required concentrations (external calibration: 0.01, 0.05, 0.10, 0.50, 1.0, 5.0, 10, and 25 ng/mL).
Sample Preparation
Evaluation of Background Contamination
In accordance with U.S. FDA method C-010.02, analysis was performed for water and milk samples. UHPLC-MS grade water was used to test PFAS background contamination and found to be free of the 16 analytes covered by the FDA method. The water samples (5 mL) were fortified with the isotopically labeled internal standards and were further mixed with 5 mL water, 150 µL formic acid, and 10 mL acetonitrile. After addition of the Supel™ QuE extraction salt package (55295-U), the mixture was placed on a shaker (1500 rpm for 10 minutes) and the PFAS analytes were extracted from the water phase into the organic phase. For further clean-up of complex samples like food matrices, dSPE is required. The organic layer was therefore transferred into a second tube, containing Supel™ QuE PSA/ENVI-Carb (55479-U) and shaken for 10 minutes at 1500 rpm. After centrifugation (4000x g for 10 minutes), the sample was filtered (Millex® filters, SLGNX13) and used for LC-MS/MS analysis.
Method Performance Assessment
Following the background assessment of the method using the Supel™ QuE materials, method performance was investigated using milk as an exemplary sample matrix for quantitation of PFAS in processed foods. For that purpose, 5 mL of UHT, reduced-fat (1.5%) milk were spiked at 0.5 or 2.0 ng/mL with 16 native PFAS and 8 isotopically labeled surrogate standards. The samples were analyzed using the same methodology for the presence of PFAS analytes. Extraction and purification were performed as described in FDA method C-010.02.
LC-MS/MS analysis
An Agilent 1290 Infinity II instrument coupled to an Agilent 6495C triple quadrupole mass spectrometer was used for the LC-MS/MS analysis. Analyte separation was achieved using Ascentis® Express 90 Å PFAS (15 cm x 2.1 mm, 2.7 μm, 53560-U) as analytical column. In addition, a delay column (Ascentis® Express 90 Å PFAS Delay Column, 5 cm x 3.0 mm, 2.7 μm, 53572-U), was installed after the mixing valve and before the autosampler to offset potential PFAS contamination potentially originating from the LC system (e.g., pump, tubings, fittings, filters). Polypropylene snap cap vials were used instead of standard glass vials to avoid possible PFAS adherence to the glass surface. The LC conditions used are shown in Table 1.
Results and Discussion
A chromatogram of a solvent calibration standard containing the 16 native compounds is shown in Figure 1. All 16 compounds demonstrated a lower limit of quantitation (LLOQ) of 0.01 ng/mL for the HPLC method and an LLOQ of 0.02 ng/mL in the context of the milk sample. Linear calibration curves (0.01-25 ng/mL) with R2 ≥0.99 were obtained for all PFAS analytes (Table 2).
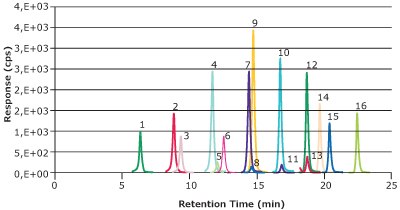
Figure 1.16 PFAS compounds at 1 ng/mL in methanol sufficiently resolved for the LC-MS/MS determination. (Peak IDs see Table 2)
The background evaluation of the FDA method C-010.02 using the recommended salt package and dSPE material showed negligible background levels for all the studied PFAS compounds (Table 3), as shown by values below the respective lower limits of quantification (LLOQ) of the LC-MS/MS method of 0.01 ng/mL (0.02 ng/mL in relation to the milk sample). Furthermore, an upfront screening of PFAS compounds in the UHPLC-MS solvents revealed concentrations below 0.01 ng/mL.
The acceptable recovery range for the investigated PFAS analytes based on the FDA guidelines for the validation of chemical methods is 40-120% (including RSD ≤ 22%) for concentrations at lower levels (i.e. 1 ng/mL). Table 4 displays the recoveries and %RSD from the experimental study where 16 compounds were spiked in quintuplicate in milk samples. All recoveries and %RSD met the requirements of the FDA method and were thus in the recommended range.
Conclusions
In this application note, the workflow for FDA method C-010.02 to analyze 16 PFAS in processed food using the QuEChERS method was investigated for milk samples. The background values of all used consumables and the LC-MS system resulted in levels below the LLOQs given in the method, thus ensuring an appropriate analysis of low levels of PFAS analytes. At both 0.5 ng/mL and 2.0 ng/mL fortified concentration levels, recoveries for all 16 compounds were well within the FDA method acceptable range of 40─120%. The calculated %RSDs were less than 11%, indicating satisfactory precision. Hence, the Supel™ QuE PSA/ ENVI-Carb clean-up tube 3, Supel™ QuE extraction salt mix (non-buffered), Ascentis® Express PFAS columns, and Millex® syringe filters proved to be suitable tools for this PFAS analysis in milk samples.
Read more about PFAS testing at SigmaAldrich.com/pfas
Discover our range of PFAS certified reference materials (CRMs) and analytical standards for reliable PFAS analysis and quantification. Click here to see the full list.
Certified Reference Materials (CRMs)
Analytical Standards
References
To continue reading please sign in or create an account.
Don't Have An Account?