Formulating, sterile filtering, and filling a drug product is complex but crucial. There are many excipient choices, filter options, and fill-finish technologies, each of which can have an impact on key attributes of the final dosage form including stability.
Final Sterile Filtration & Filling
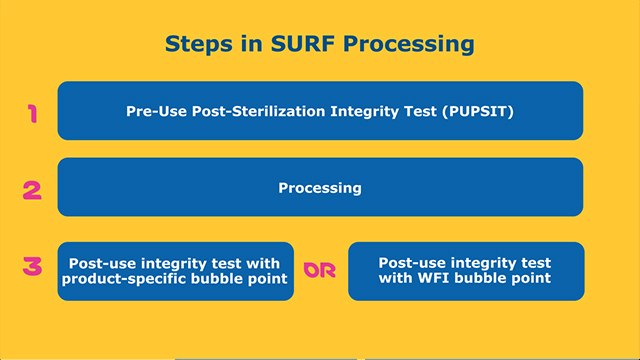
Final sterilizing filtration or bulk filtration is a critical step in the monoclonal antibody (mAb) manufacturing processes as it ensures bacterial contaminants are removed from the drug product. Multiple regulatory guidance documents describe specific considerations for filtration operations, including requirements for filter integrity testing and expectations for establishing compatibility of the filtration system with the process stream.
Final filling involves transfer of sterile filtered drug product to vials, syringes, or other containers in a highly controlled filling environment, designed to maintain sterility and mitigate any risk of product contamination.
We can support you in making informed choices for your filtration and filling operation with products and services tailored to this critical process step.
- Millipore® Filters
- Millipore® Single-Use Solutions (2D/3D single-use assemblies and storage systems, final fill assemblies, connectors)
- Emprove® Dossiers to Support Process Optimization and Safety Risk Assessment with Detailed Extractables Profile
- Millipore® Equipment & Systems (integrity tester, housings)
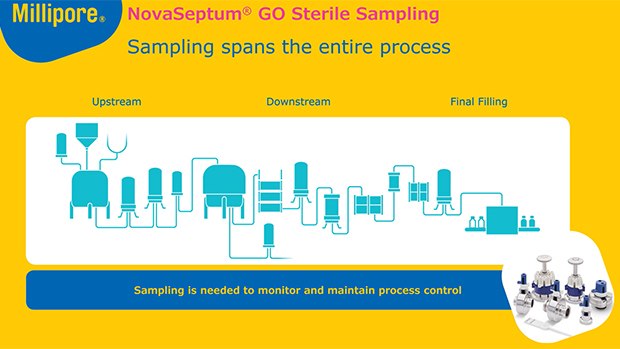
- Millipore® Sampling Solution (offline system)
- Millipore® Services (single-use and filter validation, system services, equipment installation, system qualification, system training)
- Millipore® Automation and Analytics Software
- BioReliance® Final Product Release Testing
- Millipore® CTDMO Services
Related Materials
Related Categories
Our portfolio of pharmaceutical excipients offers everything you need for medicinal drug product.
Our Viscosity Reduction Platform enables subcutaneous formulation of highly concentrated proteins.
We offer low bioburden, endotoxin materials for biopharma formulation.
The Integritest® 5 automated test instrument enables non-destructive integrity testing to be performed inline or offline on filtration devices.
Mobius® single-use systems can be designed to include either single or redundant sterilizing filters.
Robust fluid management systems mitigate the risk of process contamination. By combining our portfolio of single-use and multi-use systems, we can support your process needs.
Related Resources
- Application Note: Validation Master Plan For Filtration Systems Used In Aseptic Processing
Summarizes best practices for validating performance of critical filtration systems used in aseptic processing and details on the tests needed to meet global regulatory requirements.
- Tech note: Millipak® Final Fill Filters Reduce Contamination Risks and Simplify Filtration System Design and Operation
This tech note summarizes the results of microbial challenge studies that confirm the aseptic multi-purpose port (AMPP) prevents microbial contamination from entering the flow path.
- Application Note: Integrity Test Troubleshooting – Beyond Rewet and Retest
In this Application Note, we consider all the potential sources of test error and apply a logical approach to resolution and retesting.
- White Paper: Aseptic Process Sampling Risk Mitigation – A Regulatory Perspective
There are significant consequences associated with microbial contamination during biopharmaceutical manufacturing. Contamination increases risks for the operator, the company, and potentially the patient, all of which can result in significant negative impact.
- Application Note: Microbial Integrity of NovaSeptum® Sampling Systems upon Performance of Multiple Sampling Actuation
The NovaSeptum® and NovaSeptum® GO sampling systems are a family of products designed for single-use sterile sampling throughout the biomanufacturing process.
- Application Note: How Millipak® Barrier and Millidisk® Barrier Filters Streamline Final Sterile Filtration
These barrier filters streamline filter venting and flushing during integrity testing of critical sterilizing filters.
Explore Our Solutions
To continue reading please sign in or create an account.
Don't Have An Account?