Single-Use and Filter Validation Services
Accelerate and simplify your path to market by using our Millipore® Validation Services to select, test, and validate your filters, assemblies, and single-use systems for drug manufacturing and processing. You can rely on our experience to avoid regulatory observations and delays in your approval process.
- Proven expertise and deep knowledge of biopharmaceutical processing, process technology, and regulatory requirements for global markets
- Technology leadership with almost 50 years’ experience designing, manufacturing, and implementing filtration for pharma/biopharma manufacturing
- Global standards and localized services, with laboratories in five major geographic centers providing expertise in your time zone and language
- Commitment to the latest filtration, single-use and membrane technologies, based on proven methods and established protocols
Choosing the Right Validation Service
Helping you choose the right validation services, our validation project managers possess intimate knowledge of global and local regulations and industry best practices. We can assist you in developing and implementing an appropriate validation strategy – determining what, how, and when to test, spanning upstream, downstream, or final fill operations.
Discover an Easier Way to Choose the Right Validation Services
Upstream to the Compounding Tank/Mixing Bag
- Chemical compatibility
- Extractables/patient safety
Final Storage Single-Use Assembly
- Chemical compatibility
- Extractables/patient safety/leachables
- Non-permeation test
Final Sterilizing Filters and/or Single-Use Assembly
- Bacterial retention validation
- Bubble point/diffusion determination
- Chemical compatibility
- Extractables/patient safety/leachables
- Binding study
- Particle shedding study
- Vmax™ confirmation i.e. filter fouling evaluation
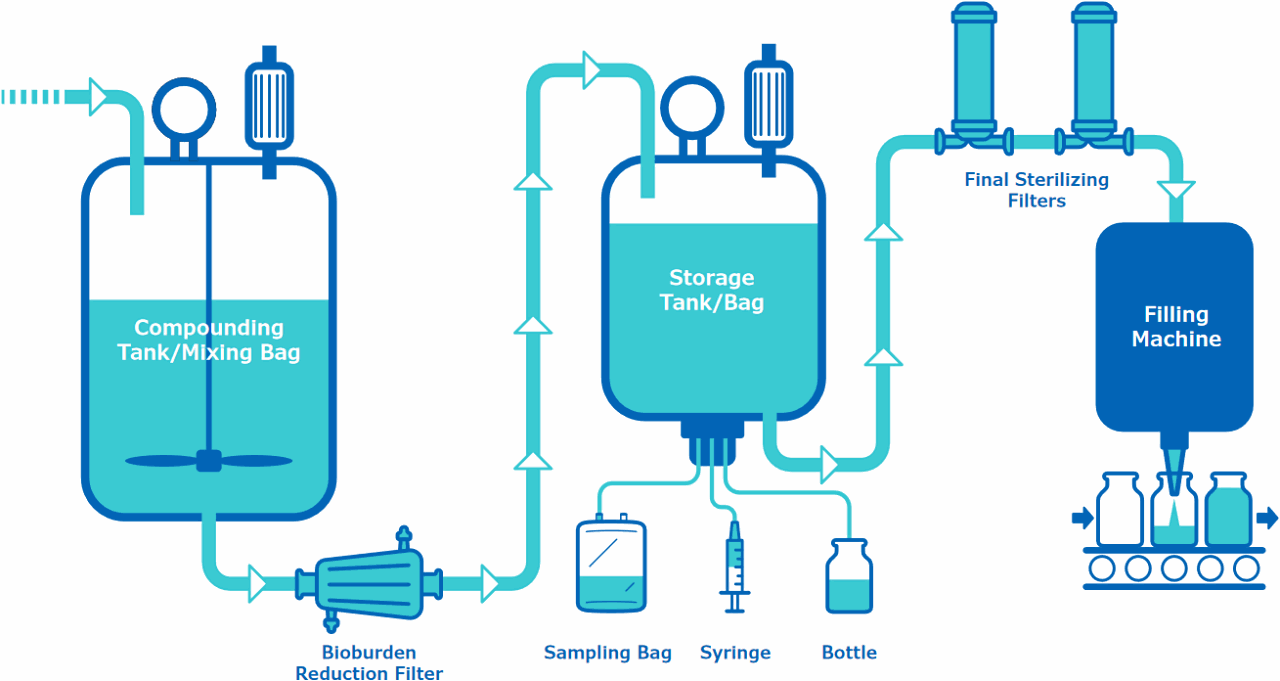
Bioburden Reduction Filter
- Chemical compatibility
- Extractables/patient safety
- Bubble point/diffusion determination
Sampling System
- Chemical compatibility
- Functionality test
- Non-permeation test
Regulatory Guidance
Recommendation, based on the pharmaceutical application and risk assessment
Trust our Global Services Network
As part of our industry-leading validation services for biopharmaceutical manufacturers, we offer these specialized capabilities at laboratories worldwide.
- Bacterial Retention Testing
- Chemical Compatibility Testing
- Filter Integrity Testing
- Extractables and Leachables
- Validation Services Consultancy
- Validation Service Levels
Related Product Resources
- Validation Services Extractables and Leachables Testing Brochure
This brochure provides details on our validation services for extractables and leachables.
- Validation Services Global Brochure
Regulatory guidance documents provide a framework to manufacturers for the production of drugs that are safe for administration to patients.
- App Note: When Do You Need to Consider Revalidating the Performance of Your Sterilizing-grade Filter?
Any product or process change that could potentially affect the cGMP compliance of a validated process or system should be evaluated and a risk assessment should be performed to evaluate the impact.
- Aseptic Validation Master Plan
Our experienced Validation Services team has summarized best practices for validating performance of critical filtration systems used in aseptic processing. If you need help, they can provide support on the various tests to meet global regulatory requirements.
- Process Development and Drug Manufacturing: Support Services
We provide comprehensive services for drug development and manufacturing, including technical and regulatory expertise and process development support.
Related Webinars
This webinar describes a scientific, risk-based approach to change management and a roadmap for biomanufacturers to expedite changes in their post-approval processes.
To continue reading please sign in or create an account.
Don't Have An Account?