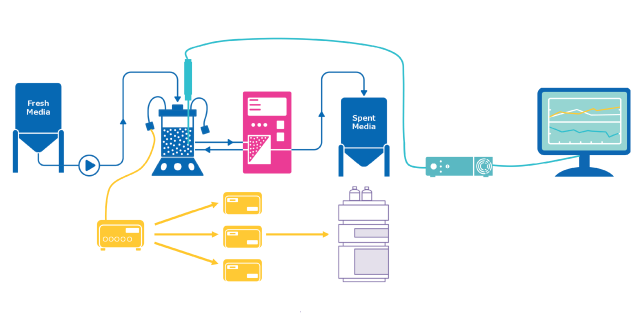
Process Analytical Technology (PAT) ensures quality in biopharmaceutical manufacturing by monitoring and controlling processes in real-time. It utilizes analytical tools to develop manufacturing processes that accommodate material and equipment variability. Once critical process parameters (CPPs) impacting critical quality attributes (CQAs) are identified, analytical methods are employed to monitor and control CPPs, maintaining them within the desired design space. This approach integrates quality by design (QbD) principles into the process rather than relying on product testing only in the end.
Real-Time Monitoring & Control in Biopharmaceuticals
Real-Time Monitoring & Control in Biopharmaceuticals

PAT Enables the Facility of the Future
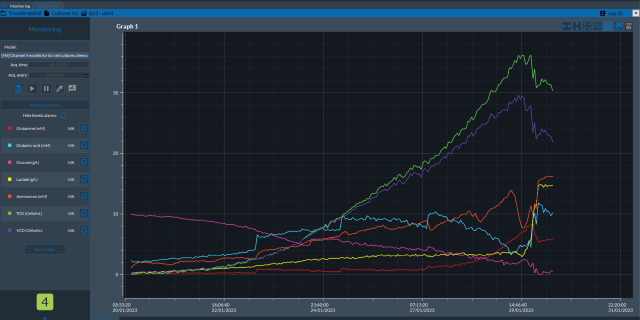
Incorporation of PAT into the manufacturing process helps establish the foundation for “bioprocessing 4.0” and the Facility of the Future, which is a complete digital transformation of pharmaceutical production using real-time monitoring, control systems, and data analytics. With PAT, real-time process monitoring is enabled, providing greater process understanding, agility, flexibility, and improved quality assurance.
Analytical Insights Improve Process Understanding and Product Quality
More recently, PAT typically involves the use of chromatographic, spectroscopic and/or mass spectrometric sensors that are integrated into upstream and downstream unit operations. These technologies are used in-line, on-line, or at-line to enable real-time monitoring and control of the process. By providing real-time insights, these sensors enable timely adjustments, optimization, and intervention, ultimately leading to improved process understanding and product quality.
Related Categories
Our off-the-shelf and customizable bioprocessing cell culture media (CCM) products enhance productivity in upstream mAb, vaccine, gene/cell therapy processes.
The Mobius® Bioreactor family includes the bench scale (2 mL and 3 L), pilot, clinical and...
Enable perfusion processes to achieve higher cell densities with the Cellicon® Cell Retention Solution.
Related Resources
- White Paper: Workforce 4.0: The Long-Term Success of the Biopharmaceutical Industry
A public-private approach to workforce 4.0 development, transitioning from conventional factories to “smart” manufacturing facilities.
- Application Note: In-Line Monitoring of CPPs and Capsid Titer in Upstream AAV Process with ProCellics™ Raman Analyzer
Explore the ProCellics™ Raman Analyzer for in-line monitoring of critical process parameters (CPPs) and capsid titer during AAV production in Human Embryonic Kidney (HEK) cell bioreactors.
- Application Note: In-line Real-time Monitoring of CHO Cell Culture Process Parameters Using Raman Spectroscopy
Cell culture processes are complex and highly variable and yet only a handful of key parameters such as temperature, pH, and dissolved oxygen (DO) are typically controlled in real time.
- Application Note: Implementation of Raman Spectroscopy for In-line Monitoring of CPPs of CHO Cell Perfusion Cultures
This application note introduces a case study for the implementation of a Raman spectroscopy soft-sensor for in-line and real-time monitoring of critical process parameters (CPP) in mammalian perfusion cell cultures.
- Application Note: Seamless Integration of Glucose Control Using Raman Spectroscopy in CHO Cell Cultures
Process analytical technology (PAT) and quality by design (QbD) are used in the biopharmaceutical industry to ensure quality is designed into a process and to achieve innovative quality improvements.
- Article: Influence of Cell Specific Parameters in a Dielectric Spectroscopy Conversion Model Used to Monitor Viable Cell Density in Bioreactors
In the biopharmaceutical industry, the use of mammalian cells to produce therapeutic proteins is becoming increasingly widespread. Monitoring of these cultures via different analysis techniques is essential to ensure a good quality product while respecting good manufacturing practice (GMP) regulations.
- White Paper: Automated Aseptic Sampling for Accelerated Access to Process and Quality Data in Upstream Bioprocessing
The biopharmaceutical market is experiencing increased demand for new medicines, reduction of costs, and new product classes, which is driving the need for increased flexibility and cost control measures in manufacturing.
- Data Sheet: MAST® Autosampling Solution
Current off-line sampling methods often present challenges related to sample source contamination, inadequate sample traceability and delivery, lengthy experimental turnaround times, and inconsistent results.
- Brochure: Process Development and Drug Manufacturing: Support Services
We provide comprehensive services for drug development and manufacturing, including technical and regulatory expertise and process development support.
Ask a PAT Expert
BioContinuum™ Platform: Your enabler of the biomanufacturing facility of the future!

To continue reading please sign in or create an account.
Don't Have An Account?