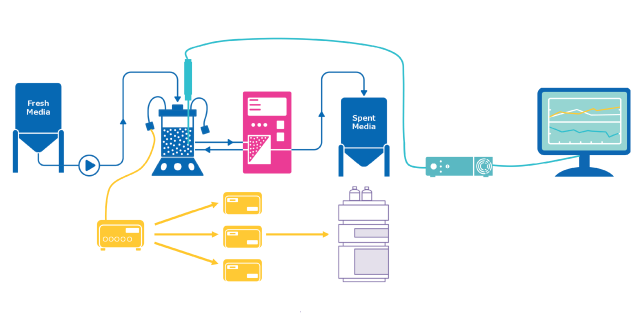
Process Analytical Technology (PAT) ensures quality in biopharmaceutical manufacturing by monitoring and controlling processes in real-time. It utilizes analytical tools to develop manufacturing processes that accommodate material and equipment variability. Once critical process parameters (CPPs) impacting critical quality attributes (CQAs) are identified, analytical methods are employed to monitor and control CPPs, maintaining them within the desired design space. This approach integrates quality by design (QbD) principles into the process rather than relying on product testing only in the end.
Automated Aseptic Sampling
Automated aseptic sampling is particularly well-suited for the development of upstream processes (USP) as it offers significant advantages for the collection of cell culture samples, but the technique can also be used in downstream processing (DSP) and microbial fermentation.
The benefits of automated aseptic sampling to process development are significant. With the usage of this PAT technique, a sample is taken from its source without manual intervention and is transported directly for analysis or storage, maintaining a closed, sterile sampling environment. Automated aseptic sampling is modality-agnostic and modular by design, offering flexible and customizable solutions in its implementation. This technology can also be combined with other PAT technologies, such as Raman spectroscopy, for the improved calibration and validation of data-driven models.
Compared to current off-line sampling and analytical procedures, automated aseptic sampling provides clear advantages including:
- Reduced contamination risk of the sample source
- Increased frequency and acquisition of process-representative data points
- Intensified turnaround times between experimentation and analytical action
- Minimized number of invariabilities associated with off-sample hold times and testing
- Decreased number of experiments required to make key process decisions

The MAST® Autosampling Solution is a versatile and modality-agnostic aseptic autosampling solution that ensures the direct collection, delivery, and analysis of samples from multiple sources without manual intervention, providing the user with more time to perform other value-added tasks.
Related Categories
Our off-the-shelf and customizable bioprocessing cell culture media (CCM) products enhance productivity in upstream mAb, vaccine, gene/cell therapy processes.
The Mobius® Bioreactor family includes the bench scale (2 mL and 3 L), pilot, clinical and...
Enable perfusion processes to achieve higher cell densities with the Cellicon® Cell Retention Solution.
Related Video
Related Resources
- Datasheet: MAST® Autosampling Solution
The MAST® Autosampling Solution is a versatile & modality-agnostic aseptic autosampling solution for reliable on-line and near real-time sample analysis.
- Technical Article: Enhance Bioprocess Sampling with MAST® Autosampling Solution
The MAST® Autosampling Solution is a cutting-edge, modality-agnostic system that automates the collection and delivery of samples from multiple sources, significantly enhancing efficiency in biopharmaceutical processes. By minimizing manual intervention, it allows scientists to focus on value-added tasks while reducing contamination risks and accelerating analytical turnaround times.
- Technical Article: Improving Efficiency and Control in Biopharma Manufacturing With Process Analytical Technology
Process Analytical Technology (PAT) is transforming biopharmaceutical manufacturing by enabling real-time monitoring and control of processes, ensuring consistent product quality amidst variability. By integrating analytical tools and Quality by Design principles, PAT enhances efficiency and accelerates the development of innovative medicines while maintaining stringent quality standards.
- Technical Article: Accelerating Data Access in Bioprocesses with Automated Aseptic Sampling
Automated aseptic sampling is an innovative process analytical technology (PAT) that significantly enhances bioprocesses in monoclonal antibody manufacturing by reducing contamination risks and increasing measurement frequency. By streamlining sampling procedures, it allows for improved efficiency, better process understanding, and tighter control, ultimately supporting real-time bioprocess monitoring and quality assurance.
- Technical Article: Accelerating Process Development with Automated Aseptic Sampling
Automated aseptic sampling is a key advancement that accelerates process development in biopharmaceutical manufacturing by enabling frequent and reliable sample collection while minimizing contamination risks. This innovative approach enhances data accessibility and process understanding, leading to more efficient workflows and improved product quality throughout the development lifecycle.
- Technical Article: Facilitating Raman Model Calibration Using Automated Sampling Technologies
This page describes the use of an innovative workflow to expedite integration of in-line Raman measurement for a bioreactor application using automated sampling and data analysis. This approach accelerates the Raman model-building phase which then enables in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during bioprocessing.
- White Paper: Automated Aseptic Sampling for Accelerated Access to Process and Quality Data in Upstream Bioprocessing
This white paper focuses on PAT and the use of automated sampling technology to accelerate analytical and quality control methods and provide an approach for access to in-line data to monitor processes in real time.
- Application Note: Enabling Accelerated Raman Model Calibration for Seamless and Reproducible Real-Time Monitoring by Combining Raman and Automated Sampling Technologies
Explore how automated sampling can accelerate the integration of a Raman analyzer for a bioreactor application, speeding up the Raman model building phase, and facilitating in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during the manufacturing process.
- White Paper: Accelerate Process Development with Automated Aseptic Sampling
This whitepaper describes evaluation of the MAST® Autosampling Solution as part of an automated PAT system implemented by Takeda Pharmaceuticals.
BioContinuum™ Platform: Your enabler of the biomanufacturing facility of the future!
To continue reading please sign in or create an account.
Don't Have An Account?