Water for Clinical Chemistry
In the clinical laboratory, water is a key factor. There are two significant reasons for the importance of water in the clinical setting: the need to comply with norms or guidelines (e.g., those of the Clinical and Laboratory Standards Institute® – CLSI®)1 and the sensitivity of the chemistries themselves to water quality. The CLSI® guideline was written to ensure the use of a basic level of water purity so that clinical chemistry assays could be run safely. Segmented into three parts, this presentation provides a comprehensive analysis of water purity requirements for various types of assays, a brief description of purification technologies and the way they can be combined for optimal effectiveness, as well as a short discussion of the role of water in the quality control process.
Section Overview
- Water Quality Requirements for the Clinical Laboratory
- Summary
- Purification Technologies
- The Role of Water in the Quality Control Process
- Selecting the Optimal Water Solution
- Related Products
Water Quality Requirements for the Clinical Laboratory
Before discussing the specific clinical chemistry applications, it should be noted that basic water requirements in the clinical laboratory include:
- Removing particulates, which can clog needles or manifolds and interfere with spectroscopic detection
- Checking silica levels to prevent the formation of deposits in the needles, which can modify the volumes dispensed
- Reducing the level of organics and the level of polyaromatic molecules such as humic and fulvic acids, which have high UV absorbance and fluorescence properties
General Chemistry, Electrolyte, Lipid and Protein Assays
General chemistry, electrolyte, lipid and protein assays seek to measure ions (e.g., Ca, K, Na, Cl) and bioorganic molecules (glucose, amino acids, lipids, etc.).
Sources of interference in these assays include:
- Ions in the feed water
- Bacteria that release ions and bioorganic molecules
These assays require water quality with:
- Low ionic content (high resistivity)
- Low bacteria count
Enzymology Assays
The purpose of enzymology assays is to measure the presence and activity of various enzymes involved in critical biochemical processes.
Sources of interference in these assays include:
- Bacteria that release enzymes and ions whose behavior is similar to the enzymes being dosed
- Ions, some of which are used as cofactors (e.g., Mg, Zn), while some are enzyme inhibitors (e.g., Cd, Pb)
- High organic concentrations, such as carboxylic acids, which may bind to some enzyme active sites and form complexes with cofactor metals
These assays require water quality with:
- Low bacteria count
- Low ionic content (high resistivity)
- Low total organic carbon (TOC)
Enzyme Immunoassays
This particularly sensitive area of immunochemistry provides critical information on various biomarkers and indicators of specific diseases (cardiology, thyroid regulation).
Sources of interference in these assays include:
- Bacteria that release enzymes whose behavior is similar to those enzymes used in amplification cascades (alkaline phosphatase, amino acid oxidase) or detection methods (alkaline phosphatase)
- Ions, some of which are used as enzyme cofactors (e.g., Mg, Zn), while others are enzyme inhibitors (e.g., Cd, Pb)
- High organic concentrations, where organics can interfere with the binding process, inhibit enzymes and interfere with fluorescence detection
These assays require water quality with:
- Low bacteria count
- Low ionic content (high resistivity)
- Low TOC
Toxicology and Therapeutic Drug Monitoring (TDM) Assays
Toxicology and TDM assays can be performed using two principal methods of analysis: immunoassay methods and chromatography. For immunoassay methods, water quality requirements are similar to the criteria previously described.
For liquid chromatography-based methods and mass spectrometry hyphenated techniques, the major requirement is a very low organics level (typically less than 5 ppb TOC). Organics can impact chromatography techniques by reducing column lifetime, causing background interference and creating ghost peaks.2,3
Trace Element Analysis
Several transition metals (e.g., Cr, Mn, Mb, Co) and heavy metals (e.g., Pb, Hg) are toxic. These metals are analyzed in occupational personal monitoring and in case of disease. The level of other elements, such as selenium or iodide, is very critical to health. Thus, reporting accurate data is particularly important.
Methods utilized include atomic absorption and ICP-MS.
Sources of interference in these assays include:
- Ions, as these are the elements being dosed
- Bacteria that release ions
These assays require water quality with:
- Very low ionic content (18.2 MΩ·cm resistivity)
- Low bacteria count
Nucleic Acid-Binding Assays
While still considered as emerging techniques, these molecular biology methods have proven to be very valuable for genetic disease identification and recognition. In general, the requirements that apply to water used in the genomics field also apply to water used by clinical laboratories performing these types of assays.4
Sources of interference in these assays include:
- Ions, e.g., phosphate and many divalent ions that can bind to the nucleic acids and interfere with the binding process
- Organic acids, in particular carboxylic acids and phosphate derivatives that can mimic nucleic acids and interfere with the binding process
- RNA and DNA
- Nucleases that would degrade the DNA and RNA being analyzed
- Bacteria, which release nucleases, DNA, RNA, ions and organic acids
These assays require water quality with:
- Low ionic level (18.2 MΩ·cm resistivity)
- Low bacteria count
- Low TOC
- Nuclease-free water (use of ultrafiltration in the purification process)
Summary
After reviewing the various tests and their sensitivity to contaminants, it is clear that ions and bacteria should be maintained at the lowest possible levels for most assays run in clinical laboratories. This is in complete accordance with CLSI® guideline recommendations (resistivity > 10 MΩ·cm, bacteria < 10 CFU/mL for Clinical Laboratory Reagent Water – CLRW). For laboratories choosing to work with Instrument Feed Water (IFW) whose specifications differ from those of CLRW, it still is highly recommended to monitor the bacterial count so that CLRW bacteria specifications are met. Some other contaminants, such as organics and bacteria by-products, also should be considered as potential pitfalls in many assays run on a routine basis.
While the CLSI® guideline recommends an organics level of not more than 500 ppb, it is advisable to decrease TOC to low levels for most assays. In some specialty chemistry (toxicology, molecular testing), the instruments and the technologies utilized require ultra low levels of TOC (< 5 to 10 ppb) and the use of Special Reagent Water (SRW). The silica concentration should be considered for its long-term impact on the instrument. Additionally, it can be beneficial to monitor the silica level on a defined basis.
Purification Technologies
A combination of purification technologies is used in the clinical laboratory setting. This technique reduces contaminant levels and also ensures that the water dispensed to the clinical analyzer is of constant quality. A description of these purification techniques and the role they play in the clinical lab follows.
- General filtration reduces the incoming particle load.
- Activated carbon is used to eliminate the oxidative agents (chlorine, chloramines, fluorine) that are present in tap water to avoid the development of microorganisms.
- Reverse osmosis (RO), a membrane-based technology that has become a standard pretreatment filtration technique, is used to decrease the load of ions, organics, colloids and particulates. However, RO rejects a percentage of the contaminants. Therefore, the quality of RO water is susceptible to variations, depending on daily and seasonal variations in tap water quality.
- To obtain a more constant water quality and eliminate variations, electrodeionization (EDI) is now included in purification systems. EDI removes ions (inorganic and organic). This technology uses selective anionic and cationic semi-permeable membrane and ion exchange (IEX) resins that are regenerated constantly with a small electrical current. No maintenance is required for this technology. Water after RO-EDI treatment has a resistivity typically > 10 MΩ·cm and a TOC level < 50 ppb (off-line measurements).
- At this stage in the purification process, water is stored temporarily in a reservoir. Depending on the assays, the clinical analyzer and the laboratory, this water can be used directly to feed the analyzer or it can be further purified. Additional purification used to reach CLRW water involves ion exchange resins (IEX), which remove ions down to a very low level.
- Bacteria control can be achieved in water purification systems and clinical analyzers with a variety of means, including screen membrane filtration (0.22 μM), germicidal UV 254 nm and chemical sanitization (peracetic acid, bleach, chlorine dioxide). Usually, a 0.22 μM membrane filter is placed at the outlet of the purification system to prevent the release of bacteria from the purification system. This final 0.22 μM filtration step is recommended by the CLSI® to produce CLRW water. UV 254 nm lamps are also used to inactivate bacteria in purified water. In-line UV as well as UV in the storage reservoir are efficient ways to control bacterial growth and to prevent buildup of biofilm in the reservoir.
- More recently, ultrafiltration has been proposed as a method of eliminating bacteria by-products (alkaline phosphatase, endotoxins) of significance in immunoassays.5
*Mechanical effects on instrument fluidic.
RO: reverse osmosis, EDI: electrodeionization, IEX resins: ion exchange resin, UF: ultrafiltration, AC: activated carbon, EIA: immuno-enzyme assay, TDM: therapeutic drug monitoring.
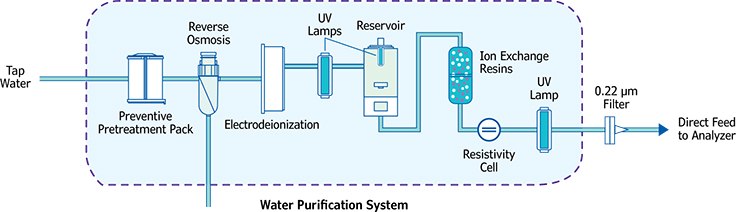
Figure 1.Illustration of the combination of technologies used to build a complete water purification system.
Typical combinations of technologies will be [RO-IEX resins-0.22 μM filter], [RO-EDI-0.22 μM filter], and [RO-EDI-IEX resins-0.22 μM filter]. As explained previously, IEX resins may be optional, and many clinical analyzers have been equipped with a purification system that does not include an EDI module. The choice of purification technologies depends on the water quality needed or selected, and on the hourly volume of water required to feed the clinical analyzer. However, it should be noted that both solutions employing EDI enable significant reduction of running costs, as less IEX resin is needed to produce water of high resistivity. The EDI module is self-regenerated and does not contribute to the running cost. For specialty chemistries, such as toxicology and nucleic acid-based assays, other purification technologies are available. Specific water purification systems that may combine IEX, activated carbon, ultrafiltration (UF) and UV photooxidation (UV185/254) usually are selected for these types of experiments.
The Role of Water in the Quality Control Process
Quality Control (QC) is one of the components in quality assurance systems. QC is used to monitor analytical processes, which detect analytical errors and prevent the report of incorrect patient values. Some pre-analytical factors (patient preparation, sample collection and handling) are difficult to monitor, as they occur outside the laboratory.
Conversely, analytical factors can be controlled and optimized to reduce the number of test failures (off-calibration, high blanks) and the release of erroneous results. Used in most assays, water is a major reagent in clinical chemistry. Water quality should be monitored as other variables, such as instrument calibration and lot-to-lot variations.
Preventive maintenance on the purification system and compliance with the CLSI® CLRW guidelines are ways to minimize issues in the assays. Avoiding poor water quality in the clinical analyzers, especially in terms of bacterial count, should be a clear focus in the overall QC system of clinical laboratories. While many laboratories comply with CLSI® recommendations, if water is stored in the clinical analyzer, this often becomes a contamination source. Lowering the bacteria count at the analyzer inlet (typically < 10 CFU/mL) reduces the contamination risk inside the analyzer and interference source in the assays.
After storage, water used in the reaction cuvettes to dilute the reagents, rinse the manifolds, tubing and needles is no longer CLSI® grade water. The on-board storage reservoir installed in the clinical analyzer usually cannot be flushed and has no UV source to reduce bacterial growth. The reservoir is not sanitized and maintained often enough, and at this stage, water quality is rarely monitored. While laboratories comply with CLSI® guidelines to ensure minimum water quality, the water often is allowed to degrade before actual use.
Selecting the Optimal Water Solution
Water impacts clinical assays in many ways. In the clinical laboratory, a good practice is to consider water as a reagent. Therefore, care should be given to the quality of the water required to run various assays in a laboratory and to water handling. Designing, selecting, and maintaining water purification units in a suitable manner can reduce issues linked to water quality in clinical assays and instrument down-time.
Pure (Type 2) Water Purification Systems
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?