Immunohistochemistry: Current Applications in Breast Cancer
Introduction
Immunohistochemistry (IHC) is used to characterize intracellular proteins or various cell surfaces in all tissues. Individual markers, or more often panels of various marker proteins, can be used to characterize various tumor subtypes, confirm tissue of origin, distinguish metastatic from primary tumor, and provide additional information which may be important for prognosis, predicting response to therapy, or evaluating residual tumor post-treatment. There is a growing list of available antibodies, which contribute to the broader utility of immunohistochemistry for solving diagnostic problems or for determining prognosis and response to therapy in breast pathology.
Benign or Malignant, That Is the Question
The most important diagnostic problems that occur in mammary gland tumor pathology are: the differential diagnosis of various types of benign lesions and carcinoma, differentiating between carcinoma in situ and invasive carcinoma, diagnosis and differentiation of microinvasion and its imitating lesions, and confirming the breast as the primary site in metastatic carcinoma.
Normal Breast Tissue
Normal glandular breast tissue is composed of three cell types which express different subsets of proteins: luminal, basal and myoepithelial. The luminal cells express cytokeratins (CK 7, -8, -18, -19), epithelial membrane antigen (EMA), milk fat globule membrane antigen (MFGM), α-lactalbumin, estrogen receptor (ER), and progesterone receptor (PR). Myoepithelial cells express basal cell type CKs (CK 5&6, -14, -17) and specific markers: smooth muscle actin (SMA), calponin, S100 and p63.1
Myoepithelial Markers: SMA, Calponin, p63, SMMHC
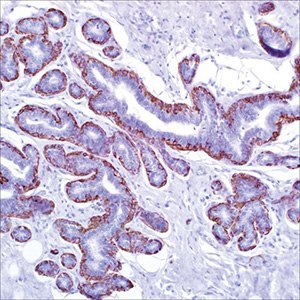
Figure 1.Calponin (CALP) on adenosis of breast
The World Health Organization’s Pathology and Genetics of Tumours of the Breast and Female Genital Organs states that ‘‘invasive breast carcinoma is a group of malignant epithelial tumours characterized by invasion of adjacent tissues and a marked tendency to metastasize to distant sites.”2 Histologically, the hallmark of invasion is the lack of myoepithelial cells (MECs), which functionally are a hybrid of both smooth muscle (‘‘myo,’’ with contractile property) and epithelial cells (with cadherin-mediated cell-cell junctions), and immunohistochemically express filamentous SMA and smooth muscle myosin as well as intermediate filaments (the epithelial keratins).
However, the presence or absence of MECs is not always easily appreciated on routine hematoxylin & eosin (H&E) sections. Throughout the years, researchers have discovered several immunomarkers that target different proteins in MECs to provide an objective measure of evaluating them when encountering challenging cases, to differentiate carcinoma in situ (CIS) (ductal [DCIS] or lobular [LCIS]) or sclerosing adenosis from invasive breast carcinoma (CA) and benign or atypical papillary lesions from papillary CAs.
The usefulness of MEC markers stems from the fact that in situ carcinomas are still contained within the ducts and lobules and, therefore, are surrounded by an MEC layer and basement membrane. Conversely, invasive carcinoma cells have already breached the MEC layer and basement membrane into the surrounding stroma. Invasive carcinomas lack the myoepithelial cell layer that normally surrounds benign breast glands. There is an exception: microglandular adenosis, a benign proliferative lesion which lacks the myoepithelial cell layer.
The partial smooth muscle phenotype expressed by MECs has resulted in the use of anti–smooth muscle proteins for their detection. These include SMA, calponin, and smooth muscle myosin heavy chain (SMMHC). All three markers share the common trait of staining myofibroblasts, which can create problems in interpretation. Myofibroblasts can be closely opposed to invasive tumor nests, creating a delusional appearance that the MEC layer is intact, resulting in a false-negative diagnosis for invasive carcinoma. Although calponin has the highest sensitivity among the three markers, SMMHC has the highest specificity, being rarely expressed in myofibroblasts.
p63, a member of the p53 gene family, is preferentially expressed in the epithelial basal cells of different organs. p63 is both a sensitive and specific MEC marker, being expressed exclusively in the nuclei of MECs without cross-reactivity with myofibroblasts. It should be noted though, that p63 is expressed in certain types of invasive breast carcinoma, such as adenoid cystic carcinomas, metaplastic carcinomas with squamous differentiation, and basal-like breast cancer. In papillary lesions, especially papillary CA, p63 may show focal patchy reactivity in tumor cells in up to 33.3% of cases. Because of its dot-like, noncontinuous nuclear-staining pattern, the evaluation may not be very easy in lesions such as sclerosing adenosis and papillary lesions. A combination of p63 and other MEC markers, such as SMMHC and calponin, is suggested.
Maspin, the product of a candidate tumor suppressor gene related to the serpin family of protease inhibitors, is intensely expressed in MECs of mammary gland. Maspin is a sensitive MEC marker with both a nuclear and cytoplasmic staining pattern and clean background without cross-reactivity to stromal myofibroblasts or vascular smooth muscle cells. However, a proportion of invasive breast CA and DCIS results are positive for maspin. According to Liu maspin expression in breast CAs and normal tissues yields the following data: (1) 100% sensitivity for MECs; (2) no cross-reactivity with myofibroblasts or vascular smooth muscle cells, as also reported by others; and (3) 29% (75 of 259) of the invasive breast CAs expressed maspin in a partial to diffuse pattern.4,6
Other MEC markers, such as SMA, p-cadherin, Wilms tumor 1 (WT1), S100, and high–molecular-weight cytokeratin (HMWCK) or basal-type cytokeratin (CK) (CK 5&6, CK 14, CK 17, CK 903) are less commonly used because of marked cross-reactivity with myofibroblasts and vascular SMA, frequent reactivity in tumor cells (HMWCK or basal-type CK, S100, p-cadherin), or low sensitivity (WT1 and S100).
Myoepithelial Cell Markers in the Evaluation of Benign Sclerosing Lesions and Adenosis
Radial scar and complex sclerosing lesion are benign lesions characterized by a proliferation of benign glands and tubules within a fibrous/fibroelastotic connective tissue stroma. Radiologically, they may present as a spiculated mass mimicking carcinoma. Histologically, the elastotic stroma may resemble desmoplasia and the entrapped glands can be confused for invasive carcinoma, especially tubular type. In these cases, IHC is necessary to highlight the MEC layer. However, as previously mentioned, MECs associated with sclerosing lesions may have different immunophenotypic characteristics from the MEC layer surrounding normal ducts and lobules. Various studies have shown loss of p63 labeling of MECs in up to 60% of cases of sclerosing adenosis. Reduced expression of CK 5&6 in 32% of cases, SMMHC in 20%, and calponin in 6%, compared to that of normal MECs was observed in one study. Therefore, caution is needed when interpreting these staining results.3,6
Microglandular adenosis is an interesting example of an adenosis lesion, which not only lacks a lobulocentric configuration but also lacks an outer MEC layer. There are reports of atypical microglandular adenoses that histologically mimic invasive carcinoma at both the architectural and cytologic level. Immunohistochemically, microglandular adenosis is negative for all MEC markers and also for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2/Neu (Her2/Neu), ‘‘the benign triple-negative lesion.’’ Immunohistochemical staining for collagen IV and laminin shows a thick layer of basement membrane around the glands. Also, the cells of microglandular adenosis are characteristically positive for S100 protein and negative for epithelial membrane antigen. The latter is a helpful feature distinguishing it from tubular carcinoma, which is positive for epithelial membrane antigen.
Collagenous spherulosis is a rare benign breast lesion, occurring as an incidental finding in 1% to 2% of surgical specimens. Histologically, this lesion is characterized by ductal proliferation arranged in a cribriform pattern. The cribriform spaces are lined by an attenuated layer of MECs and filled with a pale pink to blue-grey material. Adenoid cystic carcinoma of the breast is a rare form of breast cancer, comprising less than 1% of all cases. Histologically, these tumors are identical to their salivary gland counterparts, being composed of two cell types: cuboidal epithelial cells lining tubular duct-like structures and myoepithelial-like cells that elaborate acid mucopolysaccharides and abundant basal lamina material.
Adenoid cystic carcinoma can assume several architectural patterns including solid, trabecular, tubular, and cribriform configurations. These patterns may not be distributed homogeneously in a given tumor, causing potential diagnostic dilemma. Myoepithelial markers play an important role in differentiating these lesions from one another. In collagenous spherulosis, MEC markers stain positively at the periphery of the ducts and surrounding the lumens. In cribriform DCIS, MECs are present at the periphery only, while in invasive cribriform carcinoma, MEC staining is entirely negative. In adenoid cystic carcinoma, the pattern is interesting: while p63 staining is positive in the basal-like cells, staining with calponin and SMMHC is negative. c-kit is characteristically positive in adenoid cystic carcinoma and negative in collagenous spherulosis.
The list of MEC markers is ever growing. Although many markers are available for use, the sensitivity and specificity varies, and one should be aware of the potential pitfalls in interpretation. In an ideal world, noninvasive lesions should be decorated by a circumferential layer of MECs. In practice however, MECs associated with various pathologic lesions may exhibit immunophenotypic differences from MECs surrounding normal ducts and lobules. Although the biologic significance of this phenomenon is still uncertain, it emphasizes a few facts. First, a diagnosis of invasion should not be based on a negative result of only one MEC marker. Second, even rare staining of MECs is sufficient to rule out invasion. Lastly, the interpretation of the IHC should always be made in context of the H&E appearance. Therefore, using panels of two or more MEC markers is always recommended.3-7
Lobular or ductal carcinoma: E-cadherin, p120 Catenin, CK 8
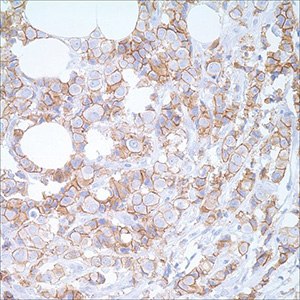
Figure 2.E-cadherin (EP700Y) on breast carcinoma
Determining whether an in situ lesion is lobular carcinoma or ductal carcinoma has clinical management implications and is another situation in which IHC is quite valuable. Generally, ductal and lobular carcinomas, either invasive or in situ, can be distinguished in H&E stained sections. In cases with non-specific morphologic characteristics, categorization can be performed through IHC; E-cadherin and p120 Catenin are currently used to differentiate between the two.
In contrast to infiltrating ductal caricnoma (IDC), which is often formed by unifocal lesions, infiltrating lobular carcinoma (ILC) is characterized by multifocality in the ipsilateral breast, seems to be more often bilateral than other types of breast cancer, and is associated with metastatic spread to unusual sites. Distant metastases from ductal carcinoma preferentially involves the lungs, liver, and brain, whereas metastases from lobular carcinoma more often involves leptomeninges, peritoneum, bone, bone marrow, and visceral organs of gastrointestinal and gynecologic tracts. The reported relative risk for contralateral carcinoma in women with ILC when compared with those with IDC ranged from 1.6 to 2. Besides the differences in the diagnostic biological markers evaluated by immunohistochemistry, ILC also differs from IDC in the risk factors, genomic profiles, and global transcription programs.8
There are some important biological behavior differences between ILC and IDC. For instance, when stratified into matched groups, ILC tends to show a worse long-term outcome with a higher incidence of development of distant metastasis, recurrences, and breast cancer mortality. Another interesting difference of these two types of breast carcinoma is the difficulty of radiologic identification, higher than ILC because of its more diffuse and multifocal breast involvement.7,8
E-cadherin, the product of the CDH1 gene (16q22.1), is a transmembrane cell adhesion molecule comprising of a acytoplasmic domain, which interacts through intracellular catenins with the actin-based cytoskeleton, and an extracellular domain, which is involved in homotypic cell-to-cell adhesion. This cadherin-catenin system is important in the organization and integrity of most epithelial tissues.3,4,7,8
Loss of E-cadherin function and/or a dysfunctional cadherin-catenin complex are common findings in lobular neoplasia, which, at a molecular genetic level, are caused by CDH1 gene aberrations with several mechanisms, such as somatic mutation of CDH1 with loss of heterozygosity on chromosome arm 16q and epigenetic changes, homozygous deletion of the CDH1 gene, and CDH1 gene promoter hypermethylation.3,7,8
Immunohistochemically, the molecular events correlate with a complete loss of expression of E-cadherin or aberrant localization of E-cadherin protein (cytoplasmic, as apical or perinuclear). Morphologically, loss or abnormal function of the E-cadherin-catenin complex determines the phenotypic features for lobular neoplasia, such as dyshesive tumor cells, diffuse infiltrative growth pattern, and a distinct metastatic pattern. Numerous studies have investigated the expression of E-cadherin protein in lobular and ductal CAs and have revealed a complete loss of E-cadherin expression in most lobular CAs, in contrast with the diffuse membranous staining for E-cadherin in most ductal CAs. However, aberrant E-cadherin expression was identified in occasional lobular CA cases, which was reported in the range of 2% to 16%.3,7,8
p120 Catenin, which was initially described as a prominent substrate of the Src oncoprotein, is encoded on band 11q11 and belongs to the catenin family. A major role of catenins (including a-catenin, b-catenin, plakoglobin, and p120 catenin) is to anchor the E-cadherin complex to the actin cytoskeleton. The a-catenin and b-catenin are complexed with the carboxy-terminal cytoplasmic tail of E-cadherin, and the p120 catenin is anchored to E-cadherin in a juxtamembranous site. p120 catenin is mostly bound to E-cadherin in the membranes, with a minor cytoplasmic pool of p120 catenin. Immunohistochemically, p120 catenin is detected in the cell membranes of a variety of epithelial and nonepithelial tissues. Shibata et al studied 60 cases of lobular CA; they found 55 cases (92%) lacked E-cadherin expression and also lost membranous staining for p120 catenin, showing instead a cytoplasmic staining pattern. In contrast, the remaining 5 cases (8%), which showed intact E-cadherin expression, were p120 catenin positive in a membranous pattern. That study suggests p120 catenin and E-cadherin are co-located in the lateral membranes of normal mammary epithelial cells, and p120 catenin is localized in the cytoplasm of E-cadherin-deficient lobular cancer cells. Several studies evaluated p120 catenin expression in non-neoplastic breast tissue, lobular neoplasia, and ductal CA and have observed intense, linear, membranous immunostaining in normal tissue and ductal carcinoma, whereas there was intense cytoplasmic immunostaining in lobular neoplasia (in situ and invasive).3,7
In addition, the differences in CKs expression may be used: HMWCK (clone 34βE12) is usually expressed by lobular carcinomas, but is absent or expressed at low levels in most cases of DCIS. In the same context, CK 8 is stained in ductal carcinoma cells in the peripheral cytoplasm, while perinuclear staining is characteristic of lobular carcinoma.3,4,8
The Evaluation of Papillary Lesions
Immunohistochemical evaluation of MECs at periphery of lesional epithelium has been used as a means to differentiate invasive from noninvasive lesions. In papillary lesions, several studies reported MEC expression in 100% of benign intraductal papillomas with or without ductal hyperplasia and 0% or focally, weakly attenuated in intracystic (encapsulated) papillary CAs. In the assessment of atypical papillary lesions (ADH/DCIS involving benign intraductal papilloma or papillary DCIS), immunohistochemical evaluation of the expression of HMWCK is a valuable adjunct, in which benign papillary lesions exhibit strong, mosaic reactivity throughout the lesion in 88% to 100% of cases, and atypical papillary lesions are nonreactive, indicating a clonal proliferation of luminal epithelial cells in 80% to 100% of cases.
Hormonal receptors, such as ER, may have additional value; in general, benign lesions show only scattered staining, whereas atypical papillary lesions are usually diffusely positive. In addition, neuroendocrine markers, such as synaptophysin and chromogranin, have been reported to be positive in most solid papillary CAs and negative in benign and atypical papillary lesions.4,5
The Evaluation of Spindle Cell Lesions
Spindle cell lesions of the breast are rare but often pose diagnostic challenges, especially in limited needle core biopsy specimens. The most common monophasic spindle cell lesions include fibromatosis, myofibroblastoma, and inflammatory myofibroblastic tumors (pseudotumor); metaplastic (sarcomatoid) CA; and the very rare primary breast sarcoma. Tumors with a biphasic pattern are mainly fibroepithelial tumors, including fibroadenoma, phyllodes tumor (PT), and biphasic metaplastic CA. Immunohistochemical studies are often employed in the workup of spindle cell lesions of breast, especially in the group with a monophasic pattern. The most important task is to exclude the potential diagnosis of metaplastic CA.
Metaplastic CA (MCA) of the breast is a heterogeneous group of neoplasms generally characterized by an intimate admixture of adenocarcinoma with dominant areas of spindle cell, squamous cell, and/or mesenchymal cell differentiation; the metaplastic spindle cell and squamous cell CAs may present in a pure form without any admixture with a recognizable adenocarcinoma. Studies show that cytokeratin immunoreactivity may be focal; therefore, a broad panel of low–molecular-weight cytokeratins, HMWCKs, and CK 14, were among the most sensitive markers to detect cytokeratin expression in this setting. CK 7, CAM 5.2 and AE1&AE3 are usually negative or only focally positive, p63 is often positive, and CD34 is nonreactive and may be used in a panel to differentiate metaplastic CA from myofibroblastoma and PT. Sex determining region Y box 10 (SOX-10) has been documented as a MEC marker in benign breast tissue and was reportedly positive in 46% (6 of 13) of MCAs in the spindle cell component. In contrast, none of the 44 cases (0%) of the fibroepithelial neoplasms, including 10 fibroadenomas and 34 PTs, were reactive.4
Fibroepithelial tumors of the breast, including fibroadenoma (FA) and PT, are a heterogeneous group of biphasic lesions combining an epithelial component and a quantitatively predominant mesenchymal component (also called a stromal component). Depending on the composition of the tumor, in cases with stromal overgrowth, especially in limited biopsy material, the epithelial component may not be easily identified. The diagnosis of metaplastic carcinoma should always be considered, and be excluded by applying a broad spectrum panel of cytokeratins and MEC markers. In addition, the histologic differentiation between FA and PT and the grading of PT are challenging on limited biopsy tissue. Investigators explored the expression of several immunomarkers in fibroepithelial tumors of the breast, attempting to identify the differential staining pattern. Among those, the Ki-67 proliferative index has been reported to be increased in borderline and especially in malignant PT, and can be useful in the classification of fibroepithelial tumors. The World Health Organization classification of PT requires greater than 10 mitoses per 10 high-power fields for malignant PT; however, no numerical cutoff has been established to define benign and borderline PTs, which were described as ‘‘few if any’’ and ‘‘moderate,’’ respectively.2 Some authors classify less than 2 mitoses per 10 high-power fields as benign PT, 2 to 5 mitoses per 10 high-power fields as borderline PT, and more than 5 mitoses per 10 high-power fields as malignant PT. Several immunomarkers showed increased expression in the stroma of malignant PT compared with that in benign tumors, including p53, CD117, epithelial growth factor receptor (EGFR), and CD10. However, no significant difference can distinguish between borderline and malignant PT. Nuclear localization of b-catenin is a common feature of fibromatoses.4 Despite the research efforts, histomorphology remains the gold standard for the diagnosis of fibroepithelial tumors.
Markers that Aid in the Identification of Breast Primary
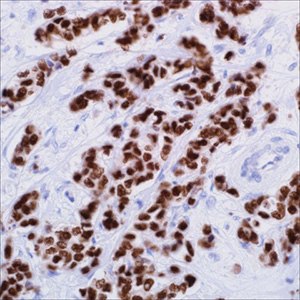
Figure 3.GATA3 (L50-823) on infiltrating ductal carcinoma of the breast
Estrogen receptor (ER), gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin A are the most commonly used breast-specific immunomarkers in the workup of tumors of an unknown primary. In metastatic breast CAs, the ER+ rate is about 50%. Both GCDFP-15 and MGB suffer low sensitivities, reported in the literature in the ranges of 35% to 55% and 65% to 70%, respectively. Data on tissue microarrays (TMAs) of 250 cases of invasive breast CAs3, including ductal, lobular, and other special types, was even lower: 30% for GCDFP-15 and 50% for MGB, which is similar to the reports by Bhargava et al who found GCDFP-15 expression in 23.1% and MGB expression in 55.4% of breast CAs, and by Lewis et al who reported GCDFP-15 labeling of 37% and MGB labeling of 54% in breast CAs. Given the frequent absence of expression of currently available breast-specific immunomarkers (such as ER, GCDFP-15, and MGB) in metastatic breast CAs, studies to discover newer breast-specific immunomarkers were undertaken. GATA binding protein 3 (GATA3) is among the most promising of immunomarkers.4
GATA3, is a member of the group of 6 zinc-finger transcription factors. It regulates the specification and differentiation of tissues, such as breast, kidney, nervous system, parathyroid gland, hair follicle, placenta trophoblasts, thymocytes, and T-cells. In normal tissues, GATA3 expression (labeling nucleus) was noted in 50% (5 of 10) of mammary gland tissues in the luminal epithelial cells in a patchy fashion, 100% (10 of 10) of the normal urothelium in a diffuse pattern, and 100% (20 of 20) of the normal renal tissues in the distal renal tubules, whereas the proximal renal tubules, glomeruli, and interstitium in normal renal tissue; the MECs in normal mammary gland tissue and 270 other varieties of normal tissues were nonreactive. Gene expression profiling studies have demonstrated that GATA3 is highly expressed in a subset of human breast tumors and that GATA3 expression in breast tumors highly correlates with expression of the ER and is seen in tumors of the ‘‘luminal A’’ subtype.4 Further immunohistochemical evaluation of GATA3 expression in 1027 varieties of CAs revealed GATA3 expression in 100% (4 of 4) of the breast ductal CAs in addition to 67% (206 of 308) of urothelial CAs but none of the other tumors tested.4
Overall, the current available data suggests that GATA3 is a sensitive and relatively specific immunomarker for breast and urothelial CAs; among the breast-specific markers available currently, GATA3 appears superior to others. However, others have subsequently reported GATA3 expression in 49% (81 of 164) of salivary gland tumors, 95% (20 of 21) of pheochromocytomas, 89% (31 of 35) of paragangliomas, 96% (24 of 25) of benign Brenner tumors of the ovary, 100% of parathyroid tumors, and 0% to 23% of squamous cell CAs of lung. In practice, when interpreting GATA3 results, caution should be exercised.
Assessment of Sentinel Lymph Node by IHC
Sentinel lymph node biopsy is now the standard of care for axillary staging in patients with invasive breast cancer. However, the protocols that are used to examine sentinel lymph nodes vary considerably between the different institutions that care for breast cancer patients. The use of cytokeratin stains to identify occult metastatic disease (isolated tumor cells) in lymph nodes that are negative on routine H&E stained sections is controversial, and the clinical significance of such findings is unclear. Recent prospective data on clinical outcomes from randomized trials for recurrence and survival based on sentinel lymph node involvement have not shown a clinical benefit from the detection of occult disease by IHC. However, cytokeratin stains may be helpful in detecting subtle metastatic involvement for certain primary tumor morphologies such as metastatic lobular carcinoma, which can be difficult to detect by routine H&E staining in some cases.5
The Role of IHC in the Evaluation of Paget Disease of the Nipple
Paget disease (PD) is a rare (1% to 4%) form of breast carcinoma characterized by the presence of malignant glandular epithelial cells within the epidermis of the nipple, with or without extension into the areola. Clinically, it presents as nipple erythema or eczema, and is sometimes associated with nipple discharge, ulceration, and inversion. Usually the finding of PD of the nipple indicates an underlying breast CA, which is mostly high-grade DCIS or IDC. In up to 13% of PD cases, no associated carcinoma is identified, implicating the role of Toker cells as the precursors of PD. Histologically, PD cells are characterized by their large size, prominent nucleoli, and abundant, pale-staining cytoplasm that may contain mucin. They can be present singly or in clusters. Although the diagnosis is usually straightforward, they can sometimes be mistaken for other cells. The occasional presence of melanin in their cytoplasm, coupled with their large size, can mimic malignant melanoma, especially if the lesion is pigmented. Toker cells resemble PD cells in having clear cytoplasm and relatively larger size than the background keratinocytes.3
Squamous cell carcinoma in situ (Bowen disease) cells can also be mistaken for PD cells. A panel of IHC markers can be helpful in these cases. PD cells are almost always positive for CK 7 and CAM 5.2, and are usually positive for Her2/Neu. Other positive staining markers with less frequency include carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), p53,GCDFP-15, and mucicarmine. ER and PR stain positively in less than half the cases. Except for Her2/Neu and mucicarmine negativity, Toker cells have the same immunoprofile as PD cells. S100 protein and HMB-45 (human melanoma black) or Melan-A positivity, coupled with negativity for epithelial markers, is diagnostic of melanoma cells. Finally, the cells in Bowen disease will be positive for pan-CK and HMWCK, positive or negative for EMA, and negative for all the remaining markers (CK 7, CEA, S100 protein, HMB-45, and Her2/Neu).3
The Role of IHC in the Evaluation of Metastases to the Breast
Although breast cancer can metastasize to many organs, the breast is not itself an attractive site for the spread of extramammary tumors. Metastatic tumors to the breast comprise approximately 1% of all tumors encountered in the breast, with a reported range of 0.4% to 1.9% in nonautopsy series. Luckily, in 70% to 80% of cases, a history of malignancy is known. However, in the remaining cases, metastases may be the initial sign of a disseminated malignancy in the patient. In adult women, malignant melanoma is considered the most common type of metastasis, followed by lung and gynecologic cancers. Proper diagnosis is essential to drive the proper systemic therapy and avoid unnecessary mastectomy. Since metastatic tumors can be very difficult to differentiate from primary tumors by histologic evaluation only, IHC testing is needed to determine identity.
The absence of an in situ component in a tumor that is negative for ER, PR, and Her2/Neu is a ‘‘red-flag’’ that the tumor may actually be a metastasis rather than a primary tumor. However, there are several pitfalls that need to be considered. First, negativity for these markers is seen in triple-negative breast cancers and therefore these features cannot exclude a breast primary tumor. Positivity for ER and PR is present in extramammary gynecologic tumors and therefore these receptor studies cannot be used to discriminate between primary breast and gynecologic metastases. Lastly, some nongynecologic tumors may express ER-a, such as lung adenocarcinomas. The percentage of positivity is related to the antibody clone used.
GCDFP-15 and mammaglobin A are probably the most specific breast markers, but they suffer from low sensitivity, thus their negativity is noninformative. A positive reaction with these markers supports evidence for a breast primary tumor, although in two reports, up to 5% of lung carcinomas expressed GCDFP-15. Furthermore, approximately half of those cases were also negative for thyroid transcription factor 1 (TTF-1). Conversely, TTF-1 immunoreactivity has recently been reported in a minority (2.4%) of breast carcinomas.
Discrimination by CKs is usually not useful because of the large number of extramammary carcinomas that are also positive for CK 7 and negative for CK 20. Nevertheless, pan-CK is extremely useful in cases of nonepithelial metastasis such as melanoma. Negativity for pan-CK, coupled with immunoreactivity to HMB-45 and Melan A, is consistent with metastatic melanoma. Positivity for S100 protein alone is of limited value since, as we previously mentioned, it can be expressed in both benign and malignant breast epithelium.
In the rare cases when serous carcinoma of the ovary/peritoneum initially presents as a breast mass with or without axillary lymphadenopathy, IHC for GCDFP-15, mammaglobin, and WT1 can be extremely useful. Immunoreactivity for WT1 antibody, together with a negative reaction for GCDFP-15 and/or mammaglobin, provides strong evidence for metastases. In a DNA microarray study of breast and ovarian carcinomas, Schaner et al have shown paired box gene 8 (PAX-8), mesothelin, and ephrin-B1 to be the best discriminators in this differential diagnosis, being highly expressed in ovarian carcinomas. Immunohistochemically, PAX-8 was shown in one study to be more sensitive and specific than WT1, being expressed in 96% of ovarian serous carcinomas versus 86% for WT1, and negative in all breast carcinoma cases as compared to 2% for WT1.3
Determining whether a breast mass presents a primary or a metastatic neuroendocrine tumor (carcinoid tumor) is very challenging, especially because the existence of a primary mammary neuroendocrine tumor is debatable. A tumor composed of nests of malignant cells with low-grade morphology, yet negative for ER and PR, should raise the suspicion for metastatic neuroendocrine tumor, and neuroendocrine markers should be used. These tumors may also resemble a solid-type invasive lobular carcinoma or LCIS, and E-cadherin is therefore very helpful in this distinction. Metastasis from a gastric signet ring cell carcinoma can closely resemble invasive lobular carcinoma. Both tumors are characterized by E-cadherin germline mutations. Therefore, immunostaining for E-cadherin is of no value. However, an IHC panel of ER, PR, and caudal-related homeobox gene 2 (CDX-2) antibodies is helpful. Gastric carcinomas are usually positive for CDX-2 and negative for ER and PR, whereas lobular carcinoma is usually negative for CDX-2 and positive for ER and PR.3
In summary, there is no magic marker yet identified that is 100% sensitive and specific for breast origin. Interpretation of such studies should be correlated with clinical history, radiologic findings, and histologic characteristics of the tumors.
Basal-like and Triple-Negative Breast Carcinoma
Approximately 12% to 24% of breast CA are categorized as triple-negative breast cancer (TNBC). TNBC is defined immunohistochemically as breast cancer that does not overexpress Her2 and is ER and PR negative. Although defined immunohistochemically, TNBCs often have radiologic and morphologic features that distinguish them from non-TNBCs.10,11
Newly diagnosed breast carcinomas in African American women are twice as likely to be triple-negative than those diagnosed in white women. TNBCs tend to behave more aggressively than non-TNBCs. Patients with TNBC tend to experience a relapse more quickly and have a higher likelihood of developing central nervous system and visceral metastases than those with non-TNBC. In a study of 1,601 women with breast cancer, 180 women with TNBC had a distant recurrence rate of 33.9% compared with 20.4% among women with non-TNBCs.10,11
Histologically, most TNBCs are classified as high grade invasive ductal carcinoma, no specific type. TNBCs are not defined by their appearance on an H&E stain but by their lack of expression of ER, PR, and Her2 on immunohistochemical staining. Additional features commonly observed in TNBCs are a perilobular lymphocytic infiltrate in breast tissue adjacent to the tumor and an intratumoral lymphocytic inflammatory infiltrate.10,11
The term basal-like breast cancer was coined to describe tumors that overexpress genes that characterize breast basal epithelial cells based on microarray gene expression assays.
Although the histologic and immunohistochemical phenotypes of TNBCs and basal-like breast cancers overlap, “triple-negative” and “basal-like” are not synonymous terms.10,11 However, because microarray gene expression assays are used mainly in the research setting, clinicians often use the triple-negative definition as a surrogate for basal-like breast cancer.
Basal-like breast cancer comprises 15% to 20% of all breast cancers and, like TNBC, tends to occur in younger premenopausal women of African American and West African descent. Basal-like cancers generally have a poor prognosis. The tumors tend to carry tumor protein p53 (TP53) mutations.
Histologically, they share features with TNBC. They are high-grade tumors with pushing borders and a stromal lymphocytic response. However, only 55% to 85% of basal-like carcinomas are triple negative on immunohistochemistry. Biomarkers expressed by basal-like breast cancers include CK 5&6, CK 14, CK 17, laminin, EGFR, fatty acid binding protein, p16, and p53.10,11 Furthermore, there is a significant number of triple-negative cancers that do not express basal markers and are classified as normal breast-like.
More than 75% of tumors arising in women carrying a germline mutation in BRCA1 have a triple-negative phenotype, a basal-like phenotype, or both. It is noteworthy that metaplastic carcinomas (1% of breast carcinomas), medullary carcinomas (3% to 5% of breast carcinomas), and salivary gland type carcinomas (2% of breast carcinomas), of the breast tend to be TNBC.
Androgen Receptor
Androgen Receptor (AR) is coexpressed with ER in the majority of breast cancers. AR inhibits proliferative activity in ER-positive tumors, but it acts independently to promote tumorigenesis in an androgen-dependent manner in ER-negative tumors. AR can be present independent of ER, with one recent review estimating that AR is expressed in approximately 30% of TNBCs. AR mediates the ligand-dependent activation of the WNT and Her2 signaling pathways, which is accomplished through direct transcriptional induction of WNT7B and HER3. Specific targeting of the AR with bicalutamide (marketed under various names including Casodex [AstraZeneca, Wilmington, DE], Cosudex, Calutide, and Kalumid) inhibited the growth of dihydrotestosterone-stimulated ER-negative/ Her2-positive tumors in vivo. Bicalutamide efficacy was tested on TNBC cell lines, of which the LAR subtype cell lines were significantly more sensitive than the other subtypes. Although the laboratory results are encouraging, the effects of AR antagonists in patients with breast cancer are currently unknown. Phase II trials with the AR antagonists bicalutamide and enzalutamide (Xtandi, Astellas Pharma, Northbrook, IL) are currently ongoing. These studies include patients with AR-positive/ER-negative/PR-negative tumors and AR-positive/TNBCs, respectively.
TNBCs are insensitive to some of the most effective therapies for non-TNBCs, which include endocrine and Her2- directed therapies. Cytotoxic chemotherapy is currently the mainstay for TNBC. Several studies have demonstrated that TNBCs have a higher pathologic complete response (pCR) rate than hormone receptor–positive breast cancers when treated with neoadjuvant chemotherapy. The efficacy of neoadjuvant therapy was conclusively demonstrated in a prospective study of one,118 patients between 1985 and 2004 at the MD Anderson Cancer Center (Houston, TX); a pCR was seen in 22% of patients with TNBC compared with 11% of patients with non-TNBC. However, patients with TNBC had significantly worse 3-year progression-free and 3-year overall survival rates, highlighting the relatively poor prognosis of this disease. This is the “triple-negative paradox.” Within all types of TNBCs, those with a high proliferative index, as measured by nuclear positivity with immunostaining for antigen identified by monoclonal antibody Ki-67, demonstrate a higher pCR but have a lower recurrence-free survival. TNBCs with a lower proliferation rate tend to have a better prognosis.10,11
Although TNBCs tend to have a good initial response to treatment, they recur more quickly than other types of breast cancer, generally within one to three years. Distant recurrence is more frequent than local recurrence, and median survival from the time of recurrence is nine months. Tumor heterogeneity may pose a confounding challenge.
HER2 Biology
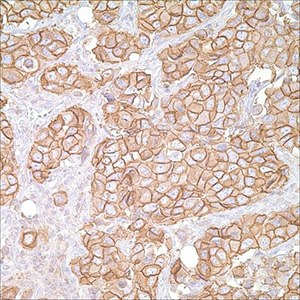
Figure 4.Her2/Neu (c-erB-2) (CB-11) on breast carcinoma
The human epidermal growth factor receptor 2 (HER2) is one of the four membrane receptor tyrosine kinases (RTKs). HER2 oncogene is a member of the human epidermal growth factor receptor family located on chromosome 17q12.1 It was first identified as a novel gene from rat neuroblastomas that transformed NIH 3T3 cells. King et al reported that DNA from human breast CA had amplification of this gene. The sequence of the neu oncogene was homologous to the erb-B oncogene and its 185kD phosphoprotein was antigenetically related to the EGFR. The other members in the epidermal growth factor receptor family are: HER1 (EGFR), HER3 (erbB3), and HER4 (erbB4). In current practice, HER2 positive rates are <20%, with most investigators currently reporting that the true-positive rate is in the range of 15% to 20%. Breast cancers can have up to 25 to 50 copies of the HER2 gene and up to a 40- to 100-fold increase in HER2 protein expression. HER2 gene amplification is responsible for HER2 overexpression in 90% of breast carcinomas.12
Prognostic Value of HER2
The value of HER2 as a prognostic factor is controversial. In a great majority of cases, abnormalities in HER2 expression at the gene or protein level have been associated with an adverse prognosis in both lymph node–negative and lymph node–positive breast cancer. Of more than 100 studies, that looked at close to 40,000 patients, 88% of the studies determined that either HER2 gene amplification or HER2 protein overexpression predicted breast cancer outcome on either univariate or multivariate analysis. In 68 of the 93 studies (73%) that utilized multivariate analysis of outcome data, the adverse prognostic significance of HER2 gene, message, or protein overexpression was independent of all other prognostic variables. In only 13 of the studies (12%) there was no correlation between HER2 status and clinical outcome identified. The majority of available data supports the view that HER2 overexpression is associated with a poorer prognosis in node-positive and node-negative breast cancer.12
HER2-targeted Therapy
Therapy targeted against HER2 includes the monoclonal antibody trastuzumab (Herceptin), and newer drugs such as small molecule tyrosine kinase inhibitor lapatinib, as well as others such as pertuzumab and ertumaxomab. The initial HER2-targeting antibody was murine, and was directed against the extracellular domain of the receptor. Subsequently a humanized version was created by inserting the antigen-binding residues of the murine antibody into a cloned human immunoglobulin G (IgG) framework. Use of Herceptin was FDA approved in 1998 for the treatment of metastatic disease. Exact mechanisms through which Herceptin exert its effects are not completely clear, but is believed to include antibody-dependent cellular cytotoxicity, disruption of downstream signaling pathway, inhibition of cell cycle progression, and antiangiogenic effects. Most response rates in clinical trials were approximately 35% with response rates varying from 12% to 68%. Responses were higher in patients who were 3+ by IHC or fluorescence in situ hybridization (FISH) positive with negligible responses in patients who were 2+ by IHC or FISH negative.12
HER2 Status and Molecular Classification of Breast Cancers
Breast cancers are molecularly classified into four main subtypes based on gene expression profiling: luminal A subtype are ER positive and/or PR positive and HER2 negative (not amplified) with low Ki-67 (<14%); Luminal B subtype are ER positive and/or PR positive and HER2 positive or if HER2 negative have high Ki-67 (>14%). These have a higher histologic grade than luminal A; HER2-amplified subtype are ER/PR negative, HER2 amplified, and more likely high grade, and Triple-negative subtype are ER, PR, and HER2 negative. A subset of the TNBCs are basal like and these express CK 5 & 6 and/or EGFR. Luminal A and B subtypes respond to endocrine therapy, but the response in the luminal B is lower and luminal B shows greater response to chemotherapy. The HER2-amplified subtype responds to Herceptin and anthracycline-based chemotherapy. The basal-like show no response to endocrine or Herceptin therapy, but seem to be sensitive to platinum-based chemotherapy, paclitaxel, and PARP inhibitors. The pathologic complete response rates in the luminal A and B subtypes is low (7% and 0%, respectively). It is much higher in the HER2-amplified and basal-like groups (45%).12
HER2 positivity seems to be lower in male breast cancer than in female breast cancer. The incidence of HER2 positivity in DCIS (24% to 38%) is higher than that for invasive breast cancer and has been associated with extensive, higher grades and DCIS with comedo-type necrosis. However, routine testing for HER2 status in DCIS is not widely performed. Hereditary breast cancer including cases that have either BRCA1 or BRCA2 germline mutations have shown a lower HER2 positivity rate. In addition, HER2 gene amplification and protein overexpression have been associated consistently with high tumor grade, high cell proliferation rate, and greater negativity for estrogen and progesterone receptors.12
To continue reading please sign in or create an account.
Don't Have An Account?