ReNcell® Human Neural Progenitors: Renewable and Consistent Human Neural Stem Cell Lines that Differentiate into Functional Neurons
ReNcell® VM and ReNcell® CX are two highly published neural stem cell lines derived from developing human brains. ReNcell® VM and CX cells are generated from the ventral mesencephalon and cortical regions of the brain, respectively, and transduced with the myc transcription factor. Both cell lines offer phenotype and genotype stability, in addition to the multipotential neuronal differentiation capacity, over long-term culture. This article describes the characteristics and differentiation of ReNcell® lines. Results of calcium and membrane potential changes in response to various ligands are also shown. The convenience to maintain in culture and flexibility to differentiate to individual scientists’ needs make ReNcell® lines the ideal platform for research and discovery.
What are Neural Stem Cells?
Neural stem cells (NSCs) were first described in the rodent brain by Reynolds and Weis in 1992. Seven years later, the isolation of human NSCs was documented by Vescovi et al. Since those initial findings, NSCs have been valuable tools for neuroscience, signal transduction and developmental biology, to name but a few.
Early studies using human NSCs were limited by the short-term stability of genotype and phenotype in culture. However, immortalization of human NSCs with the myc transcription factor has proven highly effective at overcoming these challenges. ReNcell® VM and CX lines were immortalized using myc technology. Myc is believed to drive and sustain self-renewal and proliferation of the stem cell, thus keeping differentiation at bay until desired. An up-regulation of telomerase activity is observed in myc transduced ReNcell®, which lends itself to a stable genotype in culture. Traditionally thought of as a proto-oncogene, it now appears that myc may be a “stemness” gene. An exciting discovery was made when Takahashi and Yamanaka demonstrated that a fibroblast cell could be transformed into a stem cell by using only four genes: c-Myc, Oct4, Klf4, and Sox2. This finding has since been corroborated by independent research groups.
Methods
Preparation of Laminin-Coated Flasks
We recommend coating tissue culture plastic or glassware that are used to culture ReNcell® VM cells with laminin. Tissue culture flasks should be coated on the same day that the ReNcell® VM cells are thawed from liquid nitrogen or on the same day that the cells need to be passage. The following procedure is recommended:
- Thaw the laminin in the morning at 2-8 °C. Dilute laminin with DMEM/F12 to 20 µg/mL.
- Add enough of the diluted laminin solution to cover the whole surface of the tissue culture-ware. Use 3 mL volume for 6-cm plates and 6.5 mL volume for 10-cm plates and T75 flasks. Incubate in a 37 °C, 5% CO2 incubator for at least 4 hours.
- Just before use, aspirate the laminin solution in the coated flasks and rinse the flasks once with 1X PBS.
- Prepare the Complete ReNcell® NSC Medium by adding 20 ng/mL FGF-2 and 20 ng/mL EGF (final concentrations) to ReNcell® NSC Maintenance Medium.
- Add 10 mL of the freshly made Complete ReNcell® NSC Medium to the laminin-coated T75 flasks. Incubate in a 37 °C, 5% CO2 incubator. The laminin-coated flasks are now ready to receive the cells.
Thawing of Neural Stem Cells
- Do not thaw the cells until the recommended medium and appropriately coated laminin plasticware and/or glassware are on hand.
- Remove the vial of ReNcell® VM cells from liquid nitrogen and incubate in a 37 °C water bath. Closely monitor until the cells are completely thawed. Maximum cell viability is dependent on the rapid and complete thawing of frozen cells.
IMPORTANT: Do not vortex the cells.
- As soon as the cells are completely thawed, disinfect the outside of the vial with 70% ethanol. Proceed immediately to the next step.
- In a laminar flow hood, use a 1 or 2 mL pipette to transfer the cells to a sterile 15 mL conical tube. Be careful to not introduce any bubbles during the transfer process.
- Using a 10 mL pipette, slowly add dropwise 9 mL of ReNcell® NSC Maintenance Medium (pre-warmed to 37 °C) to the 15 mL conical tube.
IMPORTANT: Do not add the whole volume of medium at once to the cells. This may result in decreased cell viability due to osmotic shock.
- Gently mix the cell suspension by slow pipetting up and down twice. Be careful to not introduce any bubbles. IMPORTANT: Do not vortex the cells.
- Centrifuge the tube at 300 x g for 3-5 minutes to pellet the cells.
- Decant as much of the supernatant as possible. Steps 4-8 are necessary to remove residual cryopreservative (DMSO).
- Resuspend the cells in a total volume of 5 mL of ReNcell® NSC Maintenance Medium (pre-warmed to 37 °C) containing freshly added 20 ng/mL FGF-2 and 20 ng/mL EGF. Note: FGF-2 and EGF should always be added fresh to the ReNcell® NSC Maintenance Medium.
- Plate the cell mixture onto the laminin-coated T75 tissue culture flask that was pre-incubated in the 37 °C incubator. The laminin-coated T75 flask should already have 10 mL of Complete ReNcell® Neural Stem Cell Medium (i.e. ReNcell® NSC Maintenance Medium containing 20 ng/mL FGF-2 and 20 ng/mL EGF).
- Incubate the cells at 37 °C in a 5% CO2 humidified incubator.
- The next day, exchange the medium with fresh ReNcell® NSC Maintenance Medium (pre-warmed to 37 °C) containing 20 ng/mL FGF-2 and 20 ng/mL EGF. Exchange with fresh medium containing FGF-2 and EGF every other day thereafter.
- When the cells are approximately 80% confluent, they can be dissociated with Accutase™ and passaged or alternatively frozen for later use.
Subculturing of Neural Stem Cells
- Prepare fresh laminin-coated flasks (refer to Preparation of Coated Flasks).
- Carefully remove the medium from the laminin-coated T75 flasks containing the confluent layer of ReNcell® VM cells.
- Rinse the flask once with 1X PBS. Note: Add the PBS slowly from the side to avoid detaching the cells.
- Aspirate the PBS.
- Apply 3-5 mL of Accutase and incubate in a 37 °C incubator for 3-5 minutes.
- Inspect the plate and ensure the complete detachment of cells by gently tapping the side of the flask with the palm of your hand.
- Apply 5 mL of ReNcell® NSC Maintenance Medium (pre-warmed to 37 °C) to the flask.
- Gently rotate the flask to mix the cell suspension. Transfer the dissociated cells to a 15 mL conical tube.
- Centrifuge the tube at 300 x g for 3-5 minutes to pellet the cells.
- Discard the supernatant.
- Apply 2 mL of ReNcell® NSC Maintenance Medium containing 20 ng/mL FGF-2 and 20 ng/mL EGF to the conical tube and resuspend the cells thoroughly. Note: Do not vortex the cells.
- Count the number of cells using a hemacytometer.
- Plate the cells to the desired density into the appropriate fresh laminin-coated flasks, plates or wells in ReNcell® NSC Maintenance Medium containing 20 ng/mL FGF-2 and 20 ng/mL EGF. We typically plated the cells at ~1.5 million cells on laminin-coated T75 flasks.
- The next day, exchange the medium with fresh ReNcell® NSC Maintenance Medium containing 20 ng/mL FGF-2 and 20 ng/mL EGF. Exchange with fresh medium containing FGF-2 and EGF every other day thereafter. The cells should be ready for passaging or harvesting 2 to 3 days after this step.
Neuronal Differentiation
- The 8-well chamber slides should be coated with 20 µg/mL laminin (please refer to the section on Preparation of Coated Flasks).
- Plate out 30,000 cells per well into an appropriately coated 8-well chamber slide in ReNcell® NSC Maintenance Medium containing 20 ng/mL FGF-2 and 20 ng/mL EGF. Total volume per well = 0.5 –0.75 mL. At this density the cells should be ~50% - 60% confluent by the next day.
Note: To prevent overgrowth of the cells by the end of the two-week differentiation protocol, it is best to avoid plating too many cells. - The next day, initiate differentiation by removing the medium from each well and replacing with ReNcell® NSC Maintenance Medium that does not contain FGF-2 and EGF. Note: Differentiation is initiated by withdrawing the growth factors so FGF or EGF should not be added to the basal medium.
- Replace with fresh ReNcell® NSC Maintenance Medium every 2-3 days for two weeks. Note: It is important that FGF or EGF not be present in the basal medium.
- After two weeks, the cells can be fixed with 4% paraformaldehyde and stained with the desired antibodies.
Results

Figure 1.ReNcell® VM Human Neural Progenitors

Figure 2. NSC Marker Expression.Both ReNcell® VM and CX human neural progenitors are grown as monolayers (A) and express NSC markers, Nestin (B, Red) and Sox-2 (B, Green). ReNcell® CX cells are able to differentiate into neurons expressing βIII-tubulin (C; Red) and glial cells expressing GFAP (D; Red). Dapi nuclear counterstain in Blue.
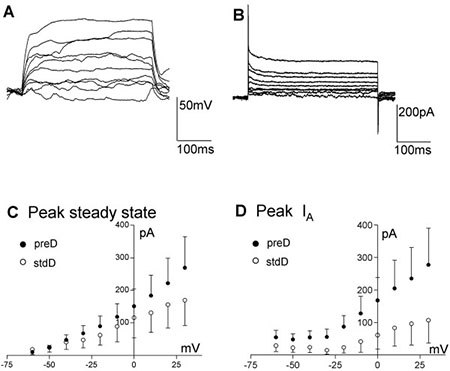
Figure 3. Electrophysiological properties of ReNcell® VM. A, B) Example of firing elicited by stepping the current command in 20 pA steps (400 ms duration) from a resting potential of ~-75 mV in ReNcell® VM 10 days after differentiation was started in control solution (A) and after adding 0.6 μM TTX (B). C, D) Same cell as in (A, B) responding with inward currents to incremental voltage steps (10 mV, from -60 mV to + 30 mV, 400 ms duration), before (C) and after (D) adding 0.6 μM TTX to the control solution. E) Average inward current-voltage relationships in control differentiated (solid squares) and after adding 0.6 μM TTX (open squares) and F) average steady-state current-voltage relationship for control (solid squares) and TTX treated (open squares).
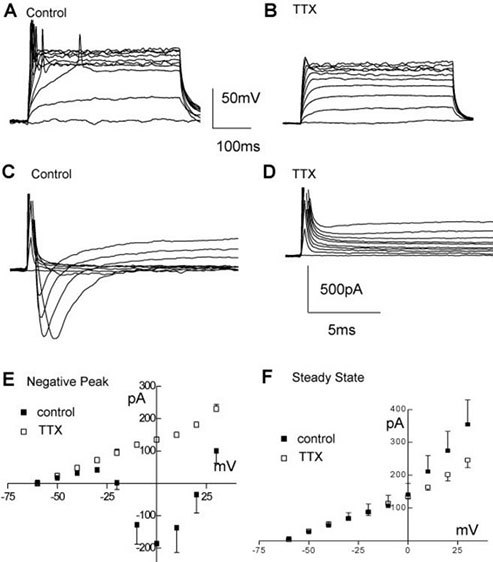
Figure 4. Electrophysiological properties of ReNcell® CX. A, B) Example of ReNcell® CX 12 days after differentiation was started using the pre-Differentiated protocol, responding to current (A; from ~-80 mV) or voltage (B) steps (10 pA or mV increments, 400 ms). C) Average voltage-current relationship for the peak current elicited by stepping the voltage from -60 to + 30 mV in 10 mV increments for the ReNcell® CX with the preD (solid circles, n = 9) or the stdD (open circles, n = 7) protocols. D) Average IA peak current for the ReNcell® CX differentiated using preD (filled circles, n = 9) or the stdD (open circles, n = 7) protocols. Despite the apparent trend there is no significant difference between these groups due to the large scatter in the data.
Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?