Membrane Scaffold Proteins: Nanodisc Formation
Read more about
- General Introduction
- Overview of Self-assembly of Target Proteins into Nanodiscs
- Core Fundamental Protocols for Nanodisc Technology
- Guidelines for Incorporation of Membrane Protein Targets into Nanodiscs
- Supplemental Protocol: Procedure for Determination of Total Phosphorus
The following material related to Nanodisc Technology is adapted from on-line content of the research group of Professor Stephen Sligar of the University of Illinois at Urbana-Champaign, with the kind permission of Professor Sligar.
The concept of membrane scaffold proteins was originally derived from the naturally occurring apoliporotein A-I component of high-density lipoprotein. Phospholipid bilayer Nanodiscs have been used to incorporate a wide variety of anchored and transmembrane proteins. Nanodiscs can provide improved solubility, homogeneity, and more native conditions over traditional microsomal preparations.
Figure 1.Membrane Scaffold
General Introduction
Membrane proteins have historically been difficult to investigate mechanistically, because many biophysical and chemical methods useful for soluble enzymes work poorly with insoluble protein aggregates. The challenge is to have a membrane protein of interest in a solubilized state for applications such as the following:
- Ease of purification
- Assay and biophysical analyses
- Crystallization for structure determination
- Biochemical manipulations that maintain the target protein in a stable state
Historically, researchers have used detergents to solubilize membrane proteins, to give mixed detergent-protein lipid micelles. However, detergents pose risks to membrane protein stability. Excess micellar phases can also interfere with many assay techniques and often have non-ideal optical properties, like absorbance and light scattering. There can also be undesired partitioning of substrates and products.
Detergents also present technical obstacles during membrane protein manipulation, because detergents often co-concentrate with the protein target and can denature or inactivate those proteins. Furthermore, many membrane protein systems require specific types of phospholipids to maintain active function. Liposome preparations have been used to incorporate membrane proteins. This approach has been useful when compartmentalization of each side of the bilayer is needed, like for assays of ion channels. Liposomes are, however, large, unstable, and difficult to prepare with precisely controlled size and stoichiometry.
Nanodisc technology offers a means to overcome some of these challenges.1-4 To prepare Nanodiscs (soluble nanoscale membrane assemblies), the membrane protein target is solubilized transiently with a detergent in the presence of phospholipids and an encircling amphipathic helical protein belt. This protein belt is referred to as a membrane scaffold protein (MSP). Upon removal of the detergent, typically via adsorption to hydrophobic beads, the target membrane protein simultaneously assembles with phospholipids into a discoidal bilayer. The MSP length controls the eventual Nanodisc size. The membrane protein finds itself in a native membrane environment, where the encircling MSP belt renders the entire assembly soluble.
Nanodisc technology has proven applicable to a wide variety of membrane proteins and protein classes, such as the following:
- G Protein Coupled Receptors (GPCR), for example, b2 Adrenergic Receptor (b2AR),5 Rhodopsin6
- Aspartate receptor (TAR receptor)7
- P450 +/- reductase8.9
- Aromatase10
- Bacteriorhodopsin (monomer / trimer)11
- Coagulation factors, e.g. Tissue Factor (TF)12
- Cholera toxin13
- SecYEG14,15
- Ion channels16
- MraY translocase17
- V-ATPase18
Our MSP products enable the incorporation of a broad range of target protein sizes. The phospholipid content will vary depending on size of the target protein incorporated in the Nanodisc. The thickness of a Nanodisc can vary depending on the type of phospholipid incorporated (typically 4.6-5.6 nm).
We offer an enhanced variety of MSP products, including MSP constructs with biotin or FLAG tags.
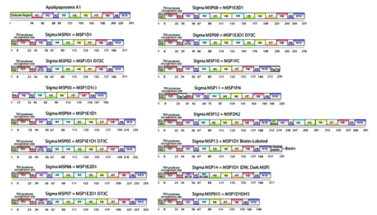
Figure 2.Membrane Scaffold Protein Constructs
Fifteen Membrane Scaffold Protein Constructs are available for applications that require tagging, chemical modifications, and accommodations for various-sized membrane proteins.
References
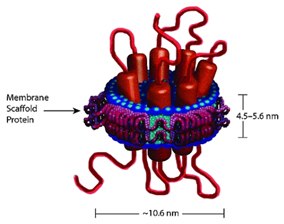
Figure 3.Nanodisc Containing 7-TM Protein
Our 15 MSP constructs allow the incorporation of a broad range of target protein sizes, generating Nanodiscs of varying diameters.
The generation of Nanodiscs involves self-assembly of their constituent parts from a detergent micellar state. The constituent parts are as follows:
- Phospholipids (PL)
- The protein of interest
- The membrane scaffold protein (MSP)
It is necessary to have enough of the correct detergent to generate this micellar starting state.1 It is likewise extremely critical for successful Nanodisc construction to have the correct stoichiometry of phospholipids (PL), scaffold protein (MSP) and target protein. Different degrees of deviation from the ideal lipid:MSP:target stoichiometry can lead to various problems with Nanodisc formation:
- Slight deviation: the result is the formation of other aggregates in the void volume, from size exclusion chromatography (SEC).
- More than slight deviation: the result is the formation of further aggregates, including discoidal aggregates.
- Extreme deviation: the result is too many other aggregates to separate or characterize. The desired nanolipoprotein particles cannot be isolated.
Many problems in the generation of homogeneous Nanodisc structures tend to result from incorrect lipid:MSP ratios. Another notable cause of problems is the use of other amphipathic helical MSP sequences which have not been optimized to produce homogeneous and monodisperse entities.2
When including a detergent-solubilized target protein in the self-assembly mixture, a general problem is that it’s not known at the outset how many PL that the target protein will displace in the assembly. In other words, the net number of PL in the resultant target protein incorporated into the Nanodiscs is not known. Although reasonable estimates are possible for how many lipids are displaced from the bare discs for some systems, like 7-transmembrane (7-TM) proteins, anchored P450s and some other systems, the best general approach is to try 3-5 different PL:MSP:target protein ratios, based on a thorough review of the literature.
One fairly common question relates to protocols that incorporate more than one protein into a Nanodisc. Several references discuss this question with respect to the assembly of bacteriorhodopsin for some phospholipids.3-6 The procedure is facilitated by the use of target proteins which have a purification tag. In general, to incorporate monomeric proteins into Nanodiscs, one strategy is to self-assemble with an excess of PL and MSP over the target. This generates bare discs, and other discs with the target. The latter can then be separated, most ideally by making use of the tag on the target protein.
Another general question of whether Nanodisc technology works for all proteins. It is certainly possible to form Nanodiscs with a great variety of membrane proteins and membrane protein assemblies. However, Nanodisc assembly will not work if the target protein is already in an aggregated soluble state. Such aggregated soluble proteins may probably be inactive, but are otherwise stable in aqueous solution by default. Interestingly, it is possible sometimes to have direct insertion into pre-formed Nanodiscs (e.g. comparably simple anchored proteins like cytochrome P450 reductase and cytochrome b5). However, in general, it is necessary to assemble integral membrane proteins from the detergent-solubilized state.
References
Core Fundamental Protocols for Nanodisc Technology
Membrane Scaffold Protein (MSP)
MSP’s in lyophilized / powder form (M6574, M7074):
Lyophilized MSP powder may be reconstituted with sterile water to a concentration of 5 mg/mL. The solution will contain the MSP in 20 mM Tris (pH 7.4), 0.1 M NaCl, and 0.5 mM EDTA. This buffer formulation is a good general “standard buffer” for MSP use. If desired, sodium azide may be added to a final concentration of 0.01% as an antimicrobial. Reconstituted MSP can be kept at 4 °C for up to several days. For long term storage of the lyophilized protein of solutions, we recommend temperatures below -20 °C.
MSP’s in solution form (MSP01, MSP02, MSP03, MSP04, MSP05, MSP06, MSP07, MSP08, MSP09, MSP10, MSP11, MSP12, MSP13, MSP14, MSP15):
The MSP concentration is determined spectrophotometrically and is reported on the Certificate of Analysis (CofA) for the specific lot of the given product number. Each MSP has its own molar extinction coefficient, reported on its specific Product Information Sheet. Each MSP solution has its own information on its solution formulation components. MSP solution products can be kept at 4 °C for up to several days. For long-term storage of the MSP solution products, storage in aliquots at or below -20 °C is recommended.
Phospholipid (PL):
Stock PL solutions are prepared in chloroform at 50-100 mM, and are stored long-term at -20 ºC in glass vials (e.g. 4 mL) with PTFE-lined screw caps. The PL concentration of the stock solution is determined by phosphate analysis. A phosphate analysis is available in the separate Supplemental Protocol titled “Procedure for Determination of Total Phosphorus”.
Hamilton syringes are recommended to measure volumes of organic solutions. Plastic (e.g. polypropylene, or PP) tips are at risk of organic solvents partly dissolving the tips, and the dissolved plastic can, in turn, contaminate the lipid solutions. Thus it is not advisable to use plastic pipette tips to dispense PL solutions in organic solvents.
Amberlite® beads, for detergent removal
We offer Amberlite® XAD-2 under several product numbers:
The beads should be washed with methanol in advance and thoroughly rinsed with water.
Preparation Of Nanodisc Reconstitution Mixtures
(a) Phospholipid:
- Dispense the desired amount of chloroform lipid stock solution into a disposable glass culture tube.
- Evaporate the solvent with a gentle stream of nitrogen gas in a fume hood. A thin film on the lower walls of the tube can be obtained by rotating the tube while holding it at an angle.
- To remove residual solvent, place the tube in a vacuum desiccator under high vacuum for at least 4 hours (usually overnight). Dried lipid should have an opaque, white appearance, and should not appear glassy.
(b) Sodium cholate:
- Add buffer containing sodium cholate to the dried lipid film. Typically, the cholate is at 2× the lipid concentration, e.g. 100 mM cholate in buffer is added to give 50 mM final phospholipid concentration.
- Vortex the tube.
- Heat the tube using hot tap water.
- Sonicate in an ultrasonic bath until the solution is clear, and no lipid remains on the walls of the tube. This is the working lipid stock for the assembly mixture.
- Calculate the volumes of detergent solubilized lipid, MSP, buffer and target protein (if applicable) necessary for the reconstitution mixture.
- Add MSP solution to cholate-solubilized phospholipid to yield the desired lipid:MSP ratio. Examples include these for MSP1 and MSP1E3:
- The final cholate concentration in the reconstitution mixture should be 12 - 40 mM.
- Supplement with standard buffer or cholate stock solution if necessary.
- Incubate the mixture at the appropriate incubation temperature for at least 15 minutes, or longer if needed.
Preparation of Nanodiscs
The self-assembly process is started upon removal of the detergent (cholate). Two general processes can be utilized to remove the cholate:
- Dialysis at the incubation temperature
- Dialyze the sample against 3 changes of buffer over 24 hours.
- The volume of buffer should be ~1000× the volume of the MSP:lipid:cholate mixture.
- Use of beads like XAD-2
- Remove most of the water from the beads immediately before use.
- Add 0.5-0.8 g of the damp beads per 1 mL of the reconstitution mixture.
- Place the suspension on an orbital shaker and incubate for the following times:
- Minimum 2 hours for DPPC or DMPC
- Minimum 4 hours for POPC
Nanodisc samples are fractionated on a column of Superdex® 200, or a pre-packed column such as a Superdex® 200 10/300 GL column, with the column equilibrated in MSP standard buffer (20 mM Tris (pH 7.4), 0.1 M NaCl, and 0.5 mM EDTA, with 0.01% sodium azide if desired), with a flow rate of 0.5 mL/min.
The upper limit for injection volume is 0.5 mL. Samples are filtered before injection and fractions collected at every minute. A sample chromatogram is shown below:
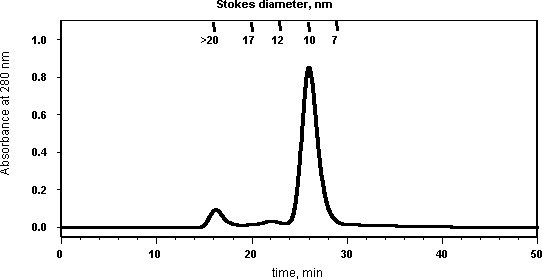
Figure 4.Sample Chromatogram
Standards (Stokes diameter in nm)
Thyroglobulin, from bovine thyroid (17 nM)
Ferritin, from horse spleen (12.2 nM)
Catalase, from bovine liver (10.4 nM)
Albumin, from bovine serum (7.1 nM)
Materials
References
Guidelines for Incorporation of Membrane Protein Targets into Nanodiscs
The following is an example of a summary general protocol to incorporate a membrane protein into POPC Nanodiscs:
- Prepare the Nanodisc reconstitution mixture following the protocol for plain Nanodiscs
- Incubate the target protein with secondary detergent.
- Mix the Nanodisc reconstitution mixture with the detergent-solubilized target protein. It is typical to use a relatively high molar ratio of MSP:target protein.
- Incubate the solution on ice for 5 minutes or longer. Ensure that the final cholate concentration in the reconstitution mixture exceeds 14 mM.
- Add washed XAD-2 beads, in a range of 0.5-1 g of wet beads per mL.
- Gently shake the mixture with an orbital shaker to keep the beads suspended for 4-18 hours.
The general methods behind membrane protein incorporation into Nanodiscs can be summarized as follows:
- Cholate-solubilized phospholipids are mixed with MSP and detergent-solubilized membrane protein.
- Everything is incubated together.
- The detergents are then removed (usually with beads), and the self-assembly occurs.
The important parameters are:
- Lipid to MSP ratio
- Temperature
- Choice of detergent
- The final concentrations of the lipid and detergent in the reconstitution mixture
The lipid:MSP ratio depends on the particular lipid and MSP.1,2
- When relatively high MSP:target protein molar ratios are used, the same lipids:MSP ratio can be used, as for “empty” nanodiscs.
- When assembly is done at low MSP:target protein ratios, it is likely that the lipids: MSP ratio will require adjustment for optimal assembly.3
The temperature should be close to the lipid melting temperature. For example, with POPC, it is advisable to conduct the assembly on ice. The lipid and detergent concentrations in the reconstitution mixture should be high enough for self-assembly to work properly. The exact value depends on the temperature, the cholate:lipid ratio, and on the presence and concentration of the second detergent. When the cholate:lipid ratio is 2:1, a suggested range of lipid concentration in the final mixture is 7 mM - 18 mM. If it is necessary to perform assembly at lower lipid concentrations, additional cholate should be introduced into the reconstitution mixture, so that the final cholate concentration will be > 14 mM.
It should be noted that the choice and concentration of the secondary detergent are entirely dependent on the membrane protein, and must be determined empirically for every new target. In particular, since it is usually not known in advance how many lipids a particular protein will displace in the fully assembled Nanodisc, titration at various lipid:MSP:target ratios is usually required. In the presence of secondary detergent, larger amounts of beads are often needed. For example, when detergents with low CMC, such as Emulgen 913 or Triton X-100, are used, a suggested range of beads is 0.8-1 g of wet beads per mL of reconstitution mixture.
One general scenario is to use excess MSP relative to target protein, and subsequently to separate Nanodiscs with incorporated membrane protein from "empty" Nanodiscs. One effective combination pairs size-exclusion chromatography (SEC) and affinity chromatography, when the membrane protein has a tag.
It must also be noted that the membrane protein must be soluble in a detergent that can be removed by dialysis and/or hydrophobic bead treatment. For instance, soluble aggregated proteins will not self-assemble into Nanodiscs Because the entire procedure can be done quickly, the target protein does not need to be stable in the detergent for very long time periods (e.g. times for standard protein purification procedures), but the target protein must be initially solubilized so that the process of self-assembly can follow. It is also potentially important to consider the speed of detergent removal in the assembly process, particularly for oligomeric proteins or situations where one wishes to incorporate a complex of membrane proteins. Thus the time of detergent removal, as modulated by quantity and hydrophobic bead treatment protocol, may need to be varied.
Another factor to note is that relatively high concentrations of glycerol interfere with the self-assembly. The final glycerol concentration in the Nanodisc reconstitution mixture should be below 3% It is acceptable, however, to add glycerol to the final Nanodisc sample.
Guidelines for Incorporation of Membrane Protein Targets into Nanodiscs
- Mix the Nanodisc reconstitution mixture with the membrane protein (which has been solubilized with suitable detergent) at the desired molar ratios. For a relatively pure target protein, it is recommended to use an excess ratio (>4) of MSP:target membrane protein. This ratio depends on the desired oligomerization state of the protein and the concentration of other membrane proteins in the target preparation. For microsomes or complex mixtures, the total membrane protein concentration should be considered also, since other proteins besides the desired target protein can also incorporate into Nanodiscs.
- If necessary, the volume of the mixture can be adjusted with Standard Buffer to ensure that the lipid concentration during Nanodisc formation is in the range of 5-20 mM. Incubate the assembly mixture for up to 2 hours at the appropriate temperature, as determined by the particular lipid being used.
- Detergent is typically removed by addition of a excess of beads.9,10 For some beads, Triton, Emulgen, and similar detergents may have sub-optimal absorption. In such cases, an increased excess of beads over the typical amount used for cholate might be needed to remove the detergent.
- The assembled Nanodiscs can be purified by affinity chromatography, such as via the 6-histidine tag for MSP’s with such a tag, or a purification method suitable for the target protein, if available. When needed, exchange the buffer prior to affinity chromatography (e.g. to remove EDTA prior to using a Ni2+ affinity column).
- Fractionate the resulting sample by size-exclusion chromatography (SEC).
- When choosing a suitable detergent to solubilize the target protein, the usual conditions call for a detergent that (a) results in soluble monomers of most or all of the target protein, and (b) allows for recovery of activity after removing the detergent. The activity does not necessarily have to be maintained while the protein is detergent-solubilized in the assembly mixture.
- Protease inhibitors can be included in the reconstitution mixture if protein degradation is a concern.
- Investigation of different detergent solubilization conditions is an important early experiment when working with target proteins under new testing for incorporation into Nanodiscs. For novel targets, information from studies with vesicles, purification methodologies, and results with related proteins, can help guide detergent selection.
References
Supplemental Protocol: Procedure for Determination of Total Phosphorus
Reagents and Equipment:
- Deionized water
- Concentrated H2SO4 (Product Number 258105)
- Ammonium molybdate(VI) tetrahydrate (Product Number A7302)
- L-Ascorbic acid
- 0.65 mM Phosphorus standard solution (Product Number P3869)
- Hydrogen peroxide (Product Number 216763)
- Hot plate or sand bath capable of temperatures >200 °C
- Aluminum block for test tubes (needed to maintain and evenly distribute heat)
- Disposable glass test tubes (13 × 100 mm)
Prepare solutions:
- 8.9 N H2SO4 solution:
- Slowly add 247 mL of conc. H2SO4 to 753 mL of deionized water.
- Allow the heat to dissipate from the 1L volumetric flask.
- Mix the solution well.
- Store the solution in a sealed container at room temperature for up to 1 year.
10% Ascorbic acid solution:
- Place 10 g of ascorbic acid into a 100 mL volumetric flask.
- Add 50 mL of deionized water. Mix the solution well.
- Dilute to 100 mL with deionized water.
- Store the solution in an amber screw-cap bottle at 4 °C for up to 1 month.
2.5% Ammonium molybdate(VI) tetrahydrate solution:
- Place 2.5 g of ammonium molybdate(VI) tetrahydrate into a 100 mL volumetric flask.
- Add 50 mL of deionized water. Mix the solution well.
- Dilute to 100 mL with deionized water.
- Store the solution in an amber screw-cap bottle at 4 °C for up to 1 month.
Prepare sample tubes:
- Place sample into the bottom of each tube (Usually 1 and 2 µL in triplicate).
- Gently remove any solvent from the tubes with N2.
- Place the following quantities of phosphorus standard into nine separate tubes:
- 0 nmol (0 µL) blank
- 16.2 nmol (25 µL)
- 32.5 nmol (50 µL)
- 48.8 nmol (75 µL)
- 65.0 nmol (100 µL)
- 81.2 nmol (125 µL)
- 97.5 nmol (150 µL)
- 113.8 nmol (175 µL)
- 130.0 nmol (200 µL)
- 162.5 nmol (250 µL)
Digestion of organic sample to inorganic phosphate:
- The samples, six standards should be labeled and placed in a vinyl-coated test tube rack.
- Add 225 µL H2SO4 to each of the standard tubes and sample tubes.
- Heat all tubes in an aluminum block in the hood at 200-215 °C for 25 min. (The temperature must be >200 °C to liberate the phosphate.)
- Remove tubes from the block, and allow them to cool for 5 minutes.
- Add 75 µL H2O2 to the bottom of all tubes.
- Replace the tubes in the block and continue to heat for an additional 30 minutes.
- The samples should be colorless at this point. (If any brown color persists, add 50 µL of H2O2 to all cooled tubes and continue heating the tubes for 15 minutes.)
- Cool tubes to ambient temperature.
- Add 1.95 mL deionized water to all tubes.
- Add 0.25 mL ammonium molybdate(VI) tetrahydrate solution to all tubes and vortex each tube to mix.
- Add 0.25 mL ascorbic acid solution to all tubes and vortex each tube to mix.
- Heat all tubes at 100 °C for 7 min.
- Cool the tubes to ambient temperature before analysis.
Spectrophotometric analysis of samples:
- Zero the spectrophotometer using the 0 nmoles standard (reagent blank).
- Determine the absorbance of each of the five standards at 820 nM.
- Determine the absorbance of each of the samples at 820 nM.
- Generate a calibration curve using the standards and determine the concentration of phosphorus in the samples.
Alternatively, a 96-well plate and a microplate reader-capable spectrophotometer can be used.
The samples should be treated as chemical waste and collected in an appropriately labeled waste container.
References
We supply the complete platform of reagents required for Nanodisc assembly and analysis.
如要继续阅读,请登录或创建帐户。
暂无帐户?