蛋白质稳定剂

无菌液体应用要求辅料具有最高的质量、纯度和可靠性。我们全面的高品质药用辅料产品组合可为您的药物产品提供所需的一切,并由 Emprove® Program简化审批准备工作并加快您的流程。当您的目标是将小批量或大批量注射剂应用成功推向市场时,您可以信赖我们的行业知识、监管专长和全面的文件,帮助您满怀信心地前进。
用于生物大分子的高品质蛋白质稳定剂
我们的高品质糖、多元醇、氨基酸和表面活性剂组合专为高风险应用而开发,可简化您在面临等关键挑战时的选择。a href="/CN/zh/technical-documents/technical-article/pharmaceutical-and-biopharmaceutical-manufacturing/downstream-processing/stabilizers and-surfactants to-prevent-protein-aggregation"> 防止生产过程中的蛋白质聚集等关键挑战时,可简化选择。优点包括
- 低内毒素和微生物限值
- 符合 ICH Q3D 的元素杂质信息
- 改进® 支持风险评估的计划和文件
- Emprove® 用于高风险应用的专家级 GMP 级产品
Products
相关产品资源
- Pharma’s Almanac: Accelerating Biologics Development with High-Quality Protein Stabilization Excipients
About protein instability and aggregation in biologic drug formulation, and how to overcome these challenges.
- Article: How to Prevent Protein Aggregation Using Stabilizers and Surfactants
Read about critical factors in long-term stability of proteins, and how different stabilizers protect against mechanical and thermal stress.
- Article: Low-in-Nanoparticulate-Impurities Sucrose for Biopharmaceutical Formulations
Read about a purification process resulting in low-NPI sucrose to achieve more stable protein formulations.
- Poster: Application of Excipients in Downstream Processing
Learn about the benefits on protein stability, chromatographic performance and viral inactivation.
- Whitepaper: Protecting Protein Stability with a Novel Grade of Sucrose
Get insights on NPIs found in commercially available sucrose, their origin and impact on protein stability.
- Whitepaper: Use of Excipients in Downstream Processing to Improve Protein Purification
Read about the effects of excipients on purification performance in Protein A chromatography and protein stability in virus inactivation.
- Whitepaper: Optimizing Poloxamer 188 for Use in Liquid Protein Formulation and Cell Culture Applications
Dive into stabilization mechanisms of poloxamer 188 and how to use attributes such as molecular weight and hydrophobicity to select the best option for liquid formulation and cell culture applications.
- Flyer: High-Quality Stabilizers for Your Biomolecules
Browse the flyer showcasing key features of our stabilizer portfolio.
- Formulation Product Finder
Quickly sort our excipients and API portfolio by dosage form, application, and many other parameters.
- Biopharmaceutical Application Guide
Explore products and services for mAb, vaccines, microbial, ADC, and plasma processes.
- Risk Mitigation Tool
Get guidance through the challenges and quality requirements of your bio-manufacturing process.
您想提高下游工艺的效率吗?
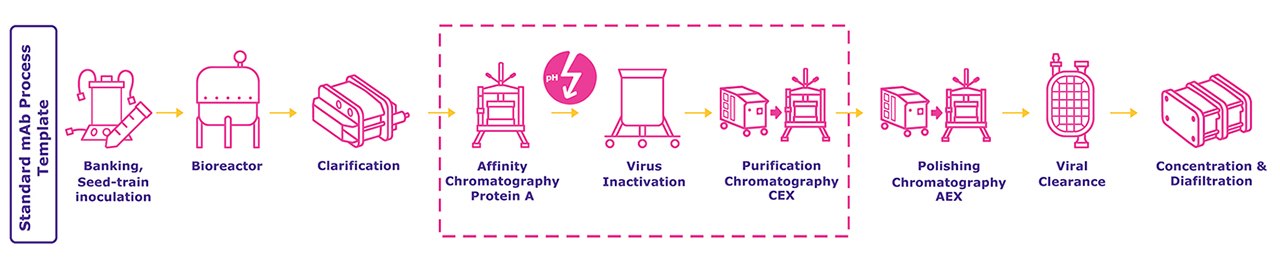
抗体和 Fc 融合蛋白的生产涉及多个下游处理单元操作。广泛使用的纯化模板包括蛋白 A 层析、低 pH 值病毒灭活以及后续的离子交换层析步骤,这些步骤大多能去除聚集体、宿主细胞蛋白和病毒等杂质,而这些杂质可能会影响产品的安全性和有效性。
蛋白 A 层析和病毒灭活过程中的低 pH 值洗脱可能会导致聚集。在这些单元操作过程中防止蛋白质聚集,而不是在随后的抛光步骤中去除多聚体,将是一种更有效的策略。想进一步了解如何通过辅料将最终产品配方中的聚集水平降至最低?点击下图查看我们的新海报"辅料在下游加工中的应用"
。如要继续阅读,请登录或创建帐户。
暂无帐户?