Charge Variant Analysis (CVA) with Strong Cation Exchange and MS-Compatible Buffers
Section Overview
Introduction
Protein therapeutics require thorough characterization to determine if all critical quality attributes (CQAs) meet specifications. This process includes the determination of various charge isoforms (variants) of the protein. Protein charge can vary based on amino acid composition, as well as from post-translational modifications (PTMs), such as deamidation, lysine clipping, N-terminal pyroglutamate formation, and glycosylation. A measure of protein charge is the isoelectric point, or pI, the pH at which the protein has an overall neutral charge. If pH is above the pI, then the protein will have a net negative charge. If below the pI, then a net positive charge will exist, and the protein can be retained on a cation exchange column.
Charge variant analysis (CVA) is often performed using cation exchange chromatography on either a weak, carboxylic acid-based resin, or a strong, sulfonic acid-based phase. In some cases, a gradient of non-volatile salts is used to affect the chromatographic separation of charge variants. With a gradient increase in cation concentration, the protein will be displaced from the resin and migrate down the column. In other cases, a mix of non-volatile organic buffers may be utilized to create a well-controlled pH gradient. In this case, the protein will elute through the column when the net charge equals zero. Neither of these approaches can be used with electrospray ionization mass spectrometry since these mobile phases are not compatible with electrospray sources.
The use of electrospray ionization with high resolution, high mass accuracy mass spectrometry, is one of the most powerful approaches to the characterization of proteins, especially when combined with chromatographic separation. Intact mass analysis of protein in “native” form refers to the analysis of nondigested protein in its natural, biological conformation. This technique is important to get an accurate picture of charge variants of the protein as they exist in biological conditions. Under denaturing conditions, such as in organic solvent or strongly acidic conditions, a protein is no longer in its native conformation, and amino acids in the interior of the protein become exposed to the solvent conditions present. This method can alter the charge and conformation of the protein so that the biological state is no longer represented in the analysis. Native MS provides spectra at a higher mass, and lower charge state, yielding a broader separation of peaks in the spectral charge envelope, and may improve mass accuracy.1 One other benefit of cation exchange-native MS, with a high-resolution instrument, is that one can discern the modifications creating the variants.2
Here, we report on the use of a polymeric cation exchange column for the charge variant analysis of monoclonal antibodies using an LC-MS compatible, multi-modal gradient separation of ammonium acetate and pH.
Experimental Conditions
In this application note, we use MS-compatible buffers to separate mAb charge variants on an SCX column but with UV detection only (Table 1). The Proteomix® column used is a polymeric, nonporous particle with a sulfonic acid bonded phase.
Results and Discussion
When doing charge variant analysis by pH gradient, others have shown that the buffering capacity of the column itself can influence the gradient.3 Consequently, to minimize the influence of column buffering capacity, it was found beneficial to use shorter columns with larger particle sizes. Additionally, we have seen in the literature, as well as personal communications, suggesting that metals in the HPLC system and column hardware can have a negative impact on the separation of proteins by adsorbing to certain groups in the protein. For this application, we used an LC system designed to be free of wetted metal components in the sample flow path.
The heterogenous protein isoforms give rise to both acidic and basic variants. These impurities are typically related to the most abundant form, or “main peak”, so that one has early eluting acidic forms and later eluting basic forms (Figure 1). As the gradient proceeds, proteins elute from the column when they reach their isoelectric point, or pI.
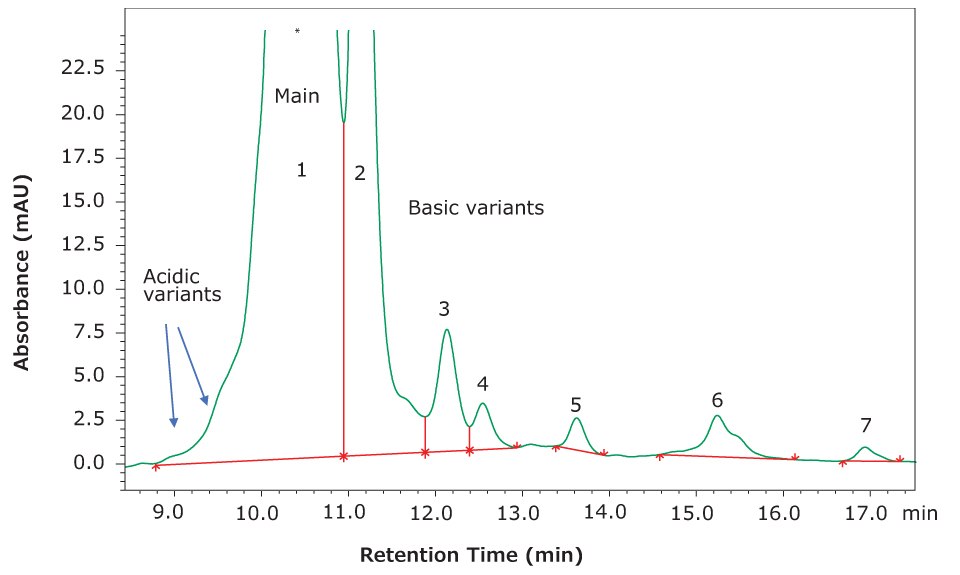
Figure 1.Separation of several variant forms of the monoclonal antibody NISTmAb.
Reproducibility
We wanted to evaluate the reproducibility of our charge variant separation over several days and using the same mobile phase preparation. The mobile phase was prepared on day 1 and after performing a series of four injections the mobile phases were left on the instrument at room temperature. On days 2 and 3, the same series of injections were made. Afterward, peaks in the region of interest were integrated and used to evaluate system reproducibility.
As shown in Figure 2, the 12 injections overlaid very well, indicating the stability of the column and mobile phase used. An analysis of the seven most abundant components of NISTmAb was used to generate the statistics in Table 2. The precision calculations of percent abundance for each component are seen to be excellent and increase only when evaluating very minor components. Retention time stability is also seen to be excellent, while recovery (based on peak area) was found to be very good, with no significant drift observed over the course of the injections.
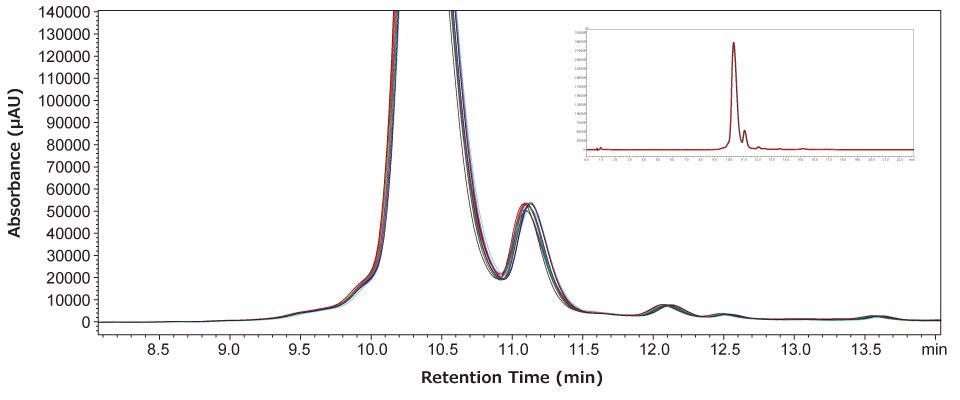
Figure 2.Overlay of 12 chromatograms analyzed over three days, four injections per day, and using the same mobile phase preparation.
In addition to our evaluation of NISTmAb, we used the same conditions and mobile phases to evaluate a separation of infliximab. This material is available as a certified reference material (CRM) at 10 mg/mL in aqueous histidine buffer. Figure 3 shows the separation achieved with infliximab using the same column and mobile phases but with a slightly modified gradient (shown in the figure inset).
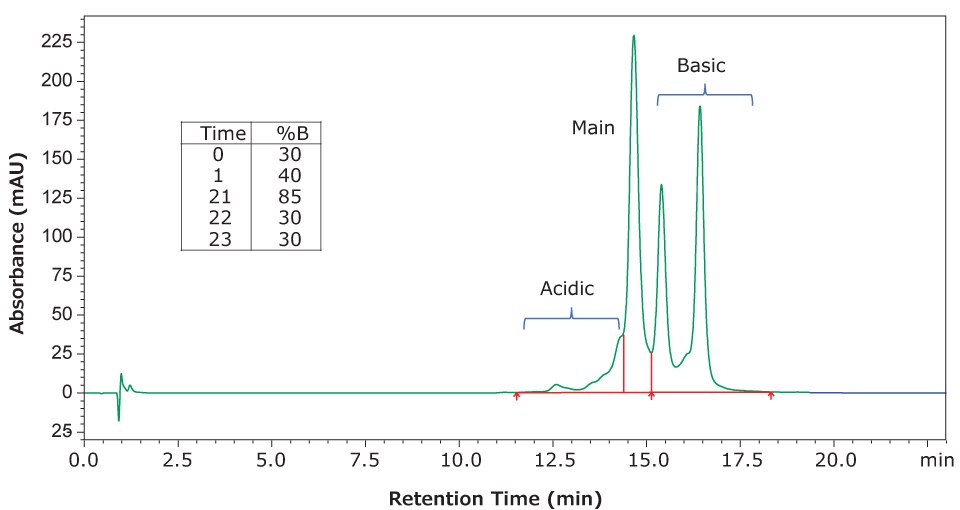
Figure 3.Separation of infliximab charge variants using gradient conditions shown in the inset and with a flow rate of 80 μL/min. All other conditions are the same as reported above. Integration of the peaks yielded 8% acidic variants (combined), 40% main peak, and 51% basic variants.
Conclusion
The Proteomix® SCX-NP5 column shows very good performance in separating acidic and basic variants in monoclonal antibodies— NISTmAb and infliximab. While mass spectrometric detection was not used here, we show the separation of charge variants using MS-compatible mobile phases (ammonium acetate buffers), as demonstrated by the literature cited below, on a metal-free UHPLC system. These mobile phases were found to provide reproducible chromatography over at least three days.
Read more about pharmaceutical analysis and quality control at SigmaAldrich.com/pharmaQC
References
如要继续阅读,请登录或创建帐户。
暂无帐户?