Applications of Iron Oxide Incorporated Conjugated Polymer Nanoparticles
- Properties of Iron Oxide Incorporated Conjugated Polymer Nanoparticles
- CPN Benefits for Diagnostic Techniques
- CPNs in Western Blot
- CPNs in Lateral Flow Rapid Diagnostics
- Summary
- Products
Properties of Iron Oxide Incorporated Conjugated Polymer Nanoparticles
Iron oxide incorporated Conjugated Polymer Nanoparticles (CPNs) are fluorescent nanoparticles with a range of unique properties that can significantly benefit many life science applications.1 Each CPN contains a semiconductor light-emitting polymer core — containing iron oxide — encapsulated within a biocompatible surfactant, permitting a range of surface chemistries for targeting molecule conjugation. CPNs demonstrate intense fluorescence across emission wavelengths ranging from 420 to 1130 nm, with consistent brightness and size (approximately 80 nm), as shown under a super-resolution microscope (Figure 1).
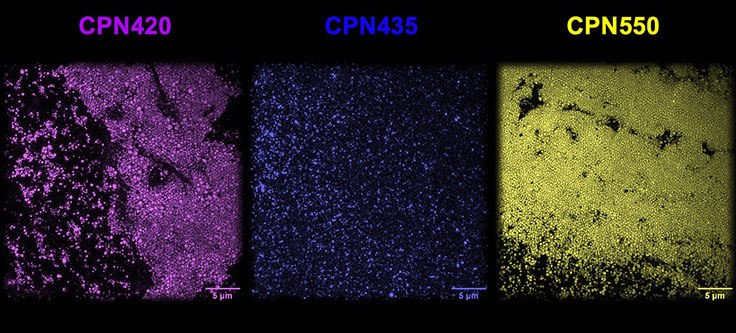
Figure 1.CPN 420 (violet), CPN 435 (indigo), and CPN 550 (yellow) imaged using super-resolution microscopy at the National Physical Laboratory, UK, displaying consistent size and brightness.
The properties of CPNs allow them to enhance a range of life science applications, where they provide benefits over conventional fluorophores. CPNs are intensely bright, with experimental data finding them 100–1000 times brighter than Q-dots and Q-beads (Figure 2). Further studies have shown that they are stable across wide ranges of temperature and pH,2 consistently emitting fluorescence over time, and showing no decrease in brightness after 24 months stored in ambient conditions (Figure 3). Also, due to their iron oxide core, CPNs can be manipulated with a magnet, offering the potential for target separation within a sample, and increased speed in assays. A range of conjugation protocols and surface chemistries are available, such as carboxyl, maleimide-thiol, alkyne click chemistry, and streptavidin for biotinylated linkage to a variety of targeting molecules, including antibodies, oligonucleotides, and receptor-ligand proteins. These features allow CPNs to be used in diagnostic techniques3 such as western blot and lateral flow, where their properties provide enhanced results over current methods.
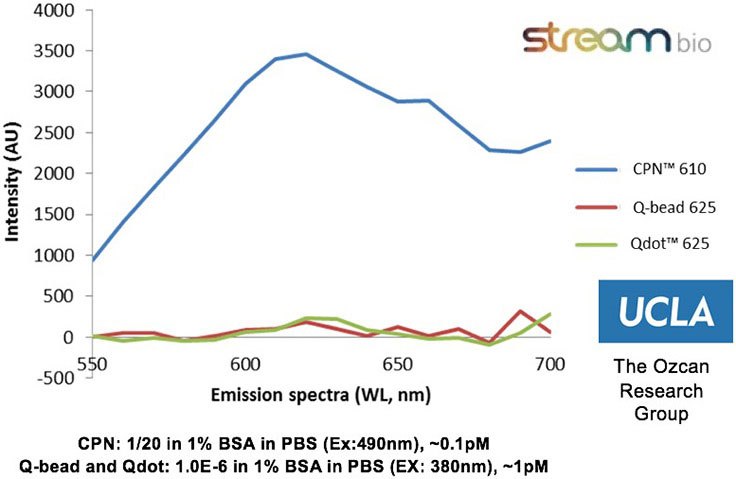
Figure 2.Experimental data from the Ozcan Research Group, University of California, Los Angeles, showing that CPN 610 (orange) is up to 100-1000x brighter than equivalent Q-beads and Q-dots. Data measured on a standard ELISA plate reader with excitation and emission optimized for each fluorophore.
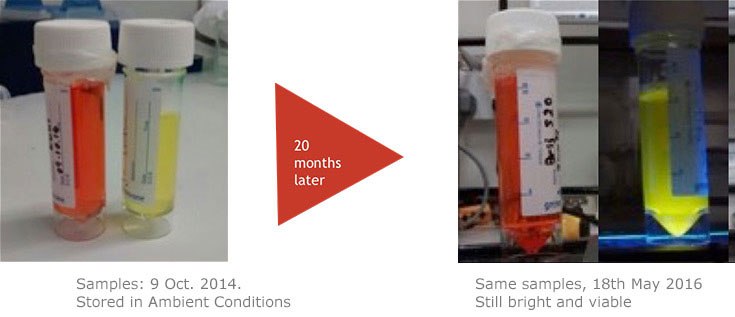
Figure 3.CPNs remain photostable for up to 24 months. As shown here, CPNs retain their fluorescence after a long period of storage.
CPN Benefits for Diagnostic Techniques
CPNs offer advantages in diagnostic techniques such as western blot, lateral flow, and ELISA due to the unique properties outlined above. As a result of their powerful brightness, even low levels of the target molecule produce a clearly visible signal, improving detection sensitivity across a wide range of assay formats and reader systems (Figure 4). Furthermore, CPNs deliver robust and stable fluorescence even at low concentrations (Figure 4) that won’t fade over time, allowing the results to be easily stored. In addition, the range of CPN emission wavelengths available supports the detection of several target molecules simultaneously, offering improved efficiency in testing. Another vital benefit of CPNs is their stability in ambient conditions, allowing for long periods of storage and use outside of the lab, crucial for point-of-care diagnostics. These properties not only increase functionality and ease of use, but they can also offer enhanced detection over diagnostic markers currently available.
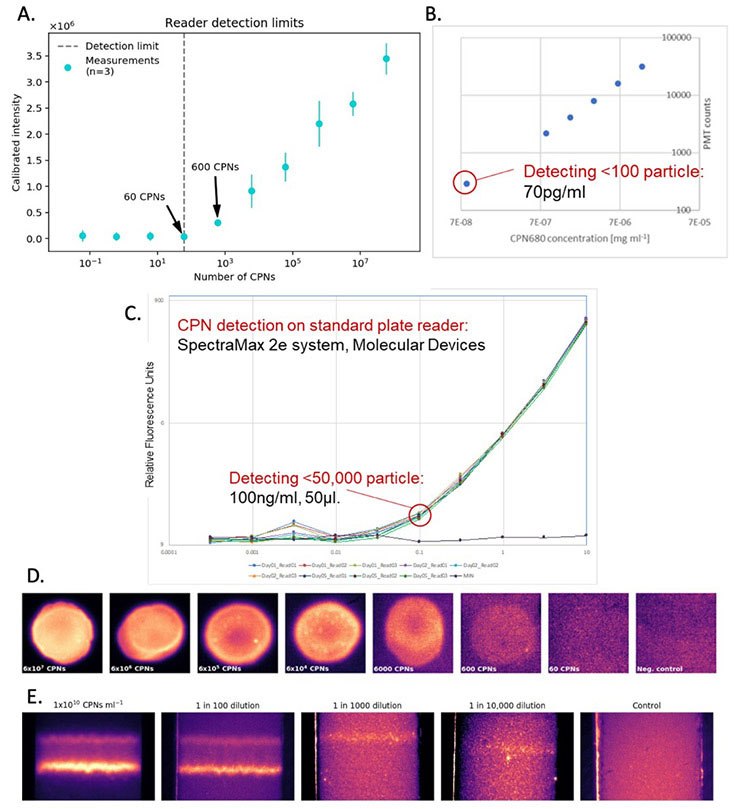
Figure 4.Detection sensitivity offered by CPNs. A. Reader detection limit based on the calibrated intensity of CPN signals at a range of CPN quantities. B. CPN fluorescence at a range of concentrations. C. CPN fluorescence at a range of concentrations, detected using a SpectraMax 2e reader system (Molecular Devices). D. CPN signal brightness from a range of quantities of CPN. E. Test line brightness at a range of CPN concentrations.
CPNs in Western Blot
CPNs can be used in western blot analyses to detect a protein of interest, as shown in Figure 5. In this example, the protein sample was prepared and transferred to the membrane with the primary antibody-linked CPNs, then incubated at room temperature for 30 minutes. CPNs are sufficiently robust to label size markers on gel, and be transferred to the membrane. The western blot signal, visualized by a gel imaging system, shows that CPNs can produce a strong and robust signal, allowing clear identification of the protein or proteins of interest, visible even after storage for extended periods.
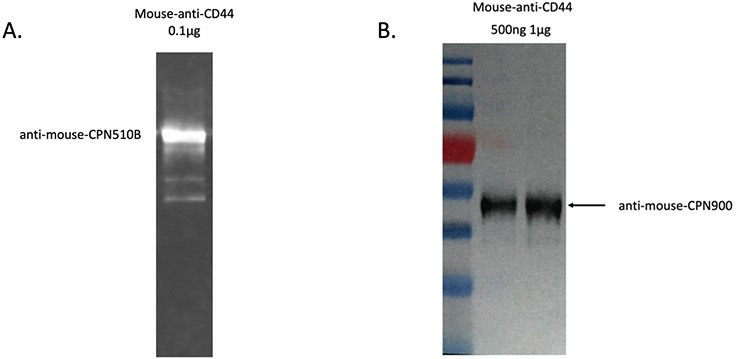
Figure 5.A. CPN western blot result using anti-mouse conjugated CPN 510B (green) to detect a mouse-anti-CD44 protein sample. B. CPN western blot result using anti-mouse conjugated CPN 900 (IR-I) to detect a mouse-anti-CD44 protein sample. Signal visualized by ChemiDoc (BioRad).
CPNs in Lateral Flow Rapid Diagnostics
The benefits of CPNs outlined above are also evident in lateral flow assays.4,5 This includes their intense brightness when compared to alternative fluorophores (Figure 6B), and the use of differently colored CPNs in multiplexed lateral flow assays (Figure 6A). These advantages can be further enhanced using a reader device, detecting a positive signal from as few as 600 CPNs (Figure 4A).
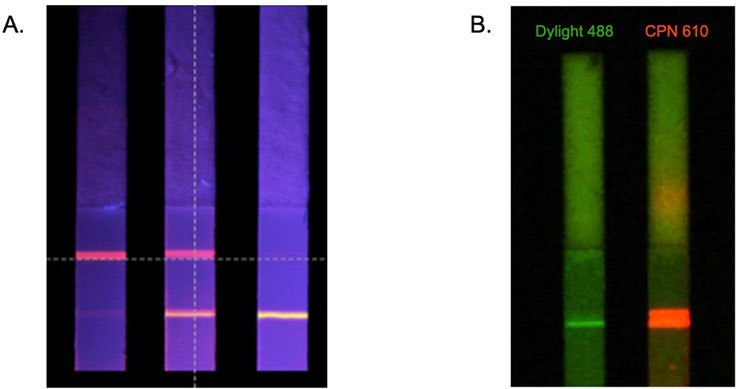
Figure 6.A. Multiplexed lateral flow detection strips using CPNs to detect two antibodies simultaneously. B. Comparison of test line brightness between CPN 610 (orange) and conventional fluorophore DyLight™ under matching conditions.
Lateral flow assays are often used in point-of-care diagnostics due to their ease of use and the rapid results they can provide.4,6 They can also produce high accuracy levels from a robust, cost-effective test, making them well-suited to widespread use outside of the lab. Based on their ability to enhance lateral flow assays and significantly increase sensitivity, CPNs have prominent applications in rapid diagnostic tests (RDTs).
The use of CPNs in RDTs may play a role in responding to many current and future healthcare challenges. By linking CPNs to the appropriate antigen, virus detection is enabled. While CPNs produce a strong visible signal without a reader, the incorporation of a reader device would facilitate PCR sensitivity levels and quantitative results (Figure 4A). In conjunction with a portable reader, CPN-based viral diagnostics may achieve sensitivity levels previously only possible within a lab. As discussed above, the unique properties of CPNs may allow the detection of low viral levels while being sufficiently robust and stable for use at a variety of locations. Work is currently underway to use this technology for a COVID-19 diagnostic test, identifying both symptomatic and asymptomatic carriers within ten minutes. In addition, the testing platform under development has the potential to detect other diseases, along with applications in agriculture and food testing.
Summary
As a result of their properties, CPNs can deliver enhanced sensitivity, improved stability, and rapid, reliable results across various life science assays, including lateral flow and western blot. This innovative technology may provide benefits in the lab and allow the development of portable, point-of-care rapid diagnostic tests to help meet current and future healthcare needs. CPNs have the power to enhance the field of rapid diagnostics, providing clear improvements over what is currently available.
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?