Application Note: Synthesis of Silver Nanowires
Uniform Ag nanowires can be readily synthesized using a modified polyol process. A typical synthesis involves ethylene glycol (EG) (Prod. No. 324558) as both the solvent and the reducing agent, with AgNO3 (Prod. No. 204390) and poly(vinvlpyrrolidone) (PVP, MW=55000) (Prod. No. 856568) as the Ag precursor and the polymeric capping agent, respectively. In this synthesis, CuCl2 (Prod. No. 203149) species can be added to facilitate the anisotropic growth of Ag nanowires. In a typical synthesis, 5 mL of EG was added to a disposable glass vial with a Teflon stir bar in it; the vial was then suspended in an oil bath (temperature = 151.5 oC) and heated for 1 h under magnetic stirring (260 rpm). At 1 h, 40 μL of a 4 mM CuCl2 solution in EG was injected into the heated EG. The solution was heated for an additional 15 min. 1.5 mL of a 0.147 M PVP solution in EG (concentration calculated in terms of the repeating unit) was then injected into the heated EG, followed by the addition of 1.5 mL of a 0.094 M AgNO3 solution in EG. The color of the reaction solution changed as follows: initially clear and colorless to yellow (within 1 min), to red-orange (within 3 min), to green, beginning cloudiness (within 5 min), to cloudiness, with a gradual shift from green to brown-red (within 30 min), and finally to opaque gray with wispiness indicating the formation of long nanowires (within 1 to 1.5 h). Upon formation of Ag nanowires, the reaction was quenched by cooling the reaction vial in a room temperature water bath. The products were washed with acetone once and water three times to remove excess EG and PVP prior to characterization.
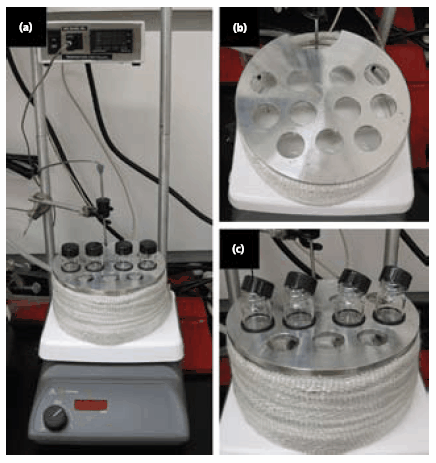
Figure 1.Experimental setup used to prepare Ag nanowires. (a) A photograph of the entire experimental setup. A temperature-controlled stirring hotplate is used, and the reaction is performed in a disposable glass vial suspended in an oil-containing crystallization dish. (b) A photograph of the custom-made vial holder, designed to rest on top of the oil-containing crystallization dish and hold up to eleven reaction vials. (c) A close-up photograph of the reaction vials. The reaction vials are supported by an O-ring and suspended in a heated oil bath. The caps are tiled off-angle during initial preheating to allow water vapor to escape.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?