Thermoelectric Performance of Perovskite-type Oxide Materials
Global Energy Challenge
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions. Meanwhile, the global energy trend appears to show only an increasing demand for energy. Alternative and environmentally benign sources of primary energy do exist. However, development is ongoing for strategies to convey these alternative sources into desired energy forms that are adequately competitive with conventional technologies. In addition to alternative sources, improvement in the efficiency of energy-conversion is considered to be a part of the solution. For example, in the mechanical conversion of heat to electricity, a major part of the primary energy is lost to the environment. Therefore, technologies for harnessing the dissipating heat component are highly desired. Direct thermoelectric power conversion, omitting any mechanical intermediate stage, is seen as one of the more promising strategies.
Thermoelectric Power Conversion
A thermoelectric generator (Figure 1) consists of an alternating series of n- and p-type conducting legs that are connected in series to the applied electric field and parallel to the heat gradient applied over the generator module. The Seebeck effect, caused by the heat gradient, accumulates the majority of the charge carriers on the cold side of each leg, thereby building up a net voltage across the electrically coupled series.
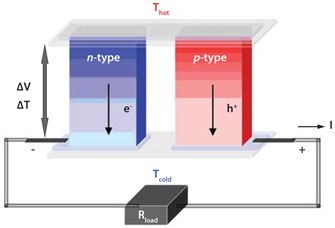
Figure 1.A schematic diagram of a thermoelectric generator, consisting of n- and p-type legs coupled electrically in series and thermally in parallel.
A good thermoelectric leg material combines a high thermopower (Seebeck coefficient, S) with a low thermal conductivity (κ) and low electrical resistivity (ρ). This is often expressed in terms of maximizing the dimensionless figure of merit ZT = S2T/ρκ, which is directly related to maximizing the efficiency of the heat-to-electricity conversion and approaching the Carnot limit at ZT → ∞.1
Since all the considered quantities deal with the charge-carrier mobility and density or the density of electronic states, achieving simultaneous improvements in all of them is challenging. As a compromise of the properties, the best-known and currently most widely applied thermoelectric materials are found among the semiconducting materials, particularly chalcogenides such as Bi2Te3 , Bi2-xSbxTe3 , and Bi2Te3-xSex.2 The chalcogenide materials already reaching the application stage present ZT values of ~1 and are also currently considered a limit for commercial interest. While the highest ZT values for any bulk thermoelectric material to date are around 1.5, reaching the efficiencies of the compressor-run heat engines still demands at least double that value.3 However, since the wasted heat generally does not have alternative uses, even the modules with ZT < 1 materials could be considered as beneficial supplements to available energy conversion technologies.
Oxides as Thermoelectric Materials
The poor chemical and physical stability under high temperatures of chalcogenides, as well as oxidizing conditions, along with relatively high toxicity, make them incompatible with major energy conversion technologies operating in air ambient conditions, e.g., combustion engines, concentrated solar radiation sources, or furnaces.4-6 Oxide materials, on the other hand, are very durable under these conditions. Although generally considered as insulators with a high Seebeck coefficient, some oxide materials are able to present ZT values that are comparable to semiconducting chalcogenides (Figure 2). Currently, the best performing oxide materials present rather low ZT ~ 0.3 in polycrystalline form.7,8 However, a better understanding of the effects of the synthesis parameters on the microstructure and on the physical properties of the materials is expected to deliver a great improvement. As an example, experiments with NaxCoO2 and [Ca2CoO3]0.62[CoO2] layered-cobalt-oxide single crystals have indicated that ZT can be enhanced up to and beyond 1.9,10 Oxide compounds, currently considered to have the most potential of all thermoelectric materials, can be divided into three main groups: layered complex oxides, doped zinc oxide derivatives, and perovskite-type oxides.
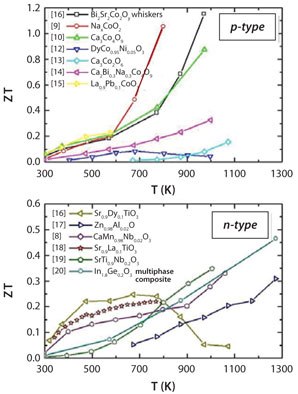
Figure 2.Figure of Merit (ZT) of selected oxide materials.
As mentioned above, the layered complex oxides, such as Na0.50CoO221 and the extended family of misfit-layered oxides [MmA2O m+2]RSq [CoO2]H (M = Co, Bi, Pb, Tl, etc.; A = Ca, Sr, Ba, etc.; m = 0, 1, 2; q ≥ 0.5),22 have presented the highest ZT values for the oxides. In addition to being relatively good electrical conductors, the main reason for their performances are their high Seebeck coefficients (80 μV K-1 < S < 170 μV K-1) when compared to the conductivity due to a large spin- and orbital entropy of the tetravalent low-spin cobalt (Co4+) in the [CoO2] layer block.23
Zinc oxide (Prod. No. 204951), also known as a transparent conducting oxide (TCO) material, has received notable attention as a potential thermoelectric material. For example, Zn0.98Al0.02O thin films have shown a reasonably high ZT ~ 0.3 at T = 1273 K.24 This is mainly due to enhancement of the electrical conductivity, which is more than three orders of magnitude higher than the value of the unsubstituted material. ZT also benefits the Seebeck coefficient by staying at rather elevated values, such as S > 100 μV K-1 at 300 K < T < 1273 K, despite the charge-carrier doping through aluminum substitution.
The major advantages of the perovskites are their profound flexibility in terms of the constituent elements covering most of the periodic table, which provides a broad selection of material-property combinations to explore. While simple perovskite-type compounds with high Seebeck coefficients are normally good insulators and, as such, not very strong potential thermoelectric materials, fruitful compromises between the lowered S and ρ are achievable through aliovalent cation substitutions. The S2/ρ factor inside the ZT equation, commonly known as the power factor, is maximized by introducing charge carriers.25-27
Thermoelectric Engineering of the Perovskite Oxide Materials
The electron-band structure of the cubic perovskite close to the Fermi level (Ef) typically consists of transition metal (TM) d-orbitals mixing with O2p orbitals. Octahedral TM-O coordination splits the TM-d orbitals into two subsets: the triply degenerate t2g orbitals and the doubly degenerate eg orbitals. The landing of the Ef at either of these orbital sets depends on the d-electron population affected by the TM valence and the TM-O charge-transfer condition.28 Among the given candidates for the thermoelectric perovskite-oxide materials, the Fermi level resides across the t2g orbitals. Consequently, the relatively high degree of spin-degeneration has an improving effect on the effective mass of a single charge carrier29 leading to the detected high Seebeck values through an elevated charge carrier entropy. The aim of the substitutions is to lower the electrical resistivity by presenting additional charge carriers at Ef, while simultaneously conserving the feasible d-orbital degeneracy. Therefore, n-type materials are doped with electrons typically through substituting lower-valent cations of the conductive TM-O network with higher-valent ones. Conversely, for the p-type cobaltates, higher valent cations need to be substituted with lower-valent ones. Ideally, an appropriate substituent should not present major changes to the crystal-structure that would undermine either the orbital degeneracy through distortions of the octahedral TM-O coordination environment or the TMd-O2p orbital overlap through lattice distortions or expansions.19
Of the substituted perovskite compounds, substituted titanates Sr1-xAxTi1-yNbyO3 (A = Ca, La, Ba, Eu),19 manganates CaMn1-xNbxO3,8 and rare-earth cobaltates LnCo1-xNixO3 (Ln = La, Pr, Nd, Sm, Gd, Dy) present the most promising ZT values.12 The n-type conducting SrTiO3 is especially known for its structural flexibility over a wide range of substitutions. The record-high ZT ~ 0.37 at T = 1000 K among perovskite oxides is measured for the SrTi0.8Nb0.2O3 thin films,30 while ZT ~ 0.35 at T = 1073 K has been achieved for a hot-pressed bulk sample of the same composition.7 However, as titanium prefers to exist in its group valence state as Ti4+, the Nb5+ substitution-induced Ti3+ tends to re-oxidize at T > 600 K, rejecting the air-operating high-temperature applications.19 Manganates, also n-type conducting, present several stable oxidation states that make them useful when considering oxidizing atmospheres.19 The record ZT ~ 0.32 at T = 1060 K for the manganates was reached with the polycrystalline CaMn0.98Nb0.02O3.8 The formation of twinned structures of orthorhombically distorted domains at low-temperatures is characteristic for the CaMnO3-based systems, while a cubic symmetry, which improves the thermoelectric properties, is realized at a high temperature range.27 Lanthanide cobaltates present the p-type conductive materials among the thermoelectric perovskites. They are well-known for their thermally activated spin-state transitions, which can be affected by the Ln substituent. The maximum ZT values for all of them are rather similar, ranging from 0.07-0.08. However, a smaller radius of the substituent cation pushes the low-temperature, low-spin to intermediate-spin and pushes the high-temperature, intermediate-spin to high-spin transitions and towards even higher temperatures, which affects the temperature of the ZT maximum. Depending on the applied temperature range, the LnCoO3 with appropriate ZT may then be chosen.
The highest ZT values, ranging between 0.3 and 0.4, are reached normally at low substitution levels (x, y < 0.2). As an extreme example, only x = 0.02 is necessary in order to reach the maximum ZT for the CaMn1-xNbxO3 system, beyond which the ZT value begins to decrease. Such a low substitution level sets requirements concerning the homogeneity of the unsintered solid-state precursor material, as spatial fluctuations with the chemical composition across the sintered body easily lead to unpredictability and instability of the properties of the sintered bodies from one synthesis batch to another. In such cases, softchemical procedures are favored, where initial mixing of the constituent cations takes place in solutions of strong organic chelating agents (e.g., EDTA, citric acid) conserving the homogeneous cation mixture over the subsequent drying, charring, and calcinations steps (Figure 3) down to the atomic scale.8
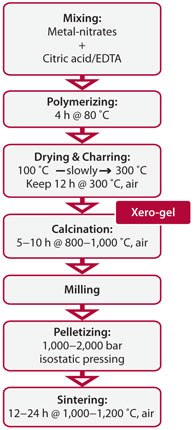
Figure 3.Flow-sheet describing a typical soft-chemical synthesis procedure for producing oxide thermoelectric materials.
While the power factor can be significantly improved through substitutions, the persistent high value of κ has hindered the ZT from reaching values much above 0.3. Substitution by heavier cations has an observable effect, but the structural distortions reducing the electronic performance tend to outweigh the benefits. In terms of thermalconductivity suppression, the shortcomings with the perovskite lattice arise from its relatively high structural isotropicity. As compared to a typical phononic mean free path length of a few hundreds of angstroms, simple perovskites are described by translational periodicities of a few angstroms. In order to improve phononic scattering, intrinsic longer-range discontinuities, interfaces, and grain boundaries with intervals approaching the phonon, mean-free path lengths should thus be introduced. Much work on the nanostructurization has been done lately,19 and several ideas, such as using layered perovskite derivatives and artificial layered superlattice structures, are currently under development or being implemented. Additionally, a nanostructurization approach aiming at a network of charge-carrier doped interfaces in an insulating matrix that presents thicknesses of only a few unit cells is expected to provide a massive improvement to the Seebeck coefficient through increased density of states at Ef due to quantum confinement. A recent highlight that proves the concept is the observation of ZT ~ 2.4 at 300 K in an artificially grown superlattice, where a single layer of SrTi0.8Nb0.2O3 is sandwiched between several layers insulating SrTiO3. A shortcoming of the approach is that the insulating matrix accounts for most of the material, thereby returning the ZT of the entire superlattice back below 0.3.31
Combining n- and p-Type Oxide Materials into a Thermoelectric Generator
Good thermoelectric conversion efficiencies require leg materials with similar physical properties. The thermoelectric compatibility factor, s = |(1+ZT)0.5-1|/(ST), describes the reduced electric current necessary for achieving the highest efficiency determined by ZT of the material.32 For maximum conversion efficiency of the thermoelectric generator, the s values of the n- and p-type materials to be combined should be as similar as possible within the operating temperature range and their ratio generally < 2. The significance of the compatibility is demonstrated as follows:
CaMn0.98Nb0.02O3 (n-type)/GdCo0.95Ni0.05O3 (p-type)
Thermoelectric properties of CaMn0.98Nb0.02O3 and GdCo0.95Ni0.05O3 materials are given in Figure 4.
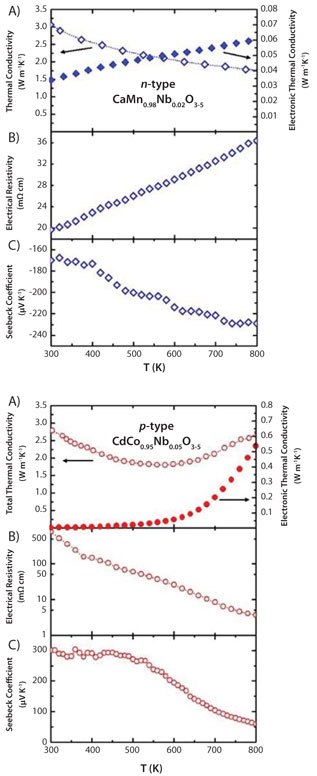
Figure 4.Temperature dependence of the thermoelectric properties A) κ, B) ρ and C) S of n-type CaMn0.98Nb0.02O3 (top panel) and p-type GdCo0.95Ni0.05O3 (bottom panel).
The κ = κph + κel of both materials (Figure 4A) are close to 3 Wm-1K-1 above 300 K. The thermal conductivity of the GdCo0.95Ni0.05O3 leg (Figure 4A, bottom) increases by 50 % and is between 600 K and 800 K; this is attributed to an increase in the electronic part (κel) due to a spinstate transition increasing the charge carrier mobility - fairly typical for the LnCoO3-based systems. On the other hand, the CaMn0.98Nb0.02O3 leg (Figure 4A, top) shows a monotonic decrease in thermal conductivity over the observed temperature scale, which is understood to arise dominantly from the phononic part of the thermal conductivity (κph).
The resistivity of CaMn0.98Nb0.02O3 (Figure 4B, top) indicates metal-like temperature behavior (dρ/dT > 0) with a steady decrease of the Seebeck coefficient, whereas semiconducting-like behavior (dρ/dT < 0) with relevantly higher resistivity is observed for GdCo0.95Ni0.05O3 (Figure 4B, bottom). The slope of the GdCo0.95Ni0.05O3 Seebeck coefficient features a down-turn also related to the spin-state transition, as described above (Figure 4C, bottom). Both materials show similar ZT values at a range of T < 600 K. However, at T > 600 K, the slope of the GdCo0.95Ni0.05O3 turns negative because the more negative slope of the Seebeck coefficient degrades the performance of the generator at T > 600 K. Furthermore, the high electrical resistivity of GdCo0.95Ni0.05O3 at T < 600 K keeps the total internal resistance of the module high and also within a lower tmperature range.
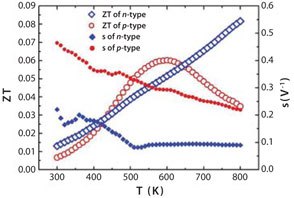
Figure 5.Figure of Merit (ZT) and thermoelectric compatibility factor (s) of the p-type GdCo0.95Ni0.05O3 and the n-type CaMn0.98Nb0.02O3 materials.
The thermoelectric compatibility factors of CaMn0.98Nb0.02O3 and GdCo0.95Ni0.05O3 are presented in Figure 5. Difference by s factor of 2-4 indicates a notably low expectable output. To overcome the difference in compatibility factors, an alternative p-type material, La1.98Sr0.02CuO4, was tested instead of the GdCo0.95Ni0.05O3 material.
CaMn0.98Nb0.02O3 (n-type)/La1.98Sr0.02CuO4 (p-type)
The thermoelectric properties of the materials are summarized in Figure 6.
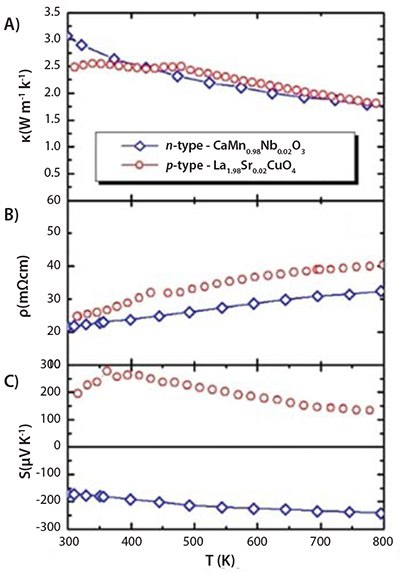
Figure 6.Temperature dependence of A) κ, B) ρ and C) S of the p-type La1.98Sr0.02CuO4 and the n-type CaMn0.98Nb0.02O3 materials.
The thermal conductivities (Figure 6A) and resistivities (Figure 6B) of both materials are similar in terms of magnitude and trend. Both appear to show metallic behavior and large Seebeck coefficients (Figure 6C).
The temperature dependence of the ZT (Figure 7) is sensitive to the Seebeck coefficient of La1.98Sr0.02CuO4, which is manifested by the degradation of the ZT value above T > 400 K. The temperature dependence of the compatibility factors (Figure 7) indicates a closer similarity (s < 2) at a lower-temperature regime (300 K < T < 500 K). At T > 500 K, a decrease in the conversion efficiency of the four-leg modules was observed. Although the gross ZT characteristics of La1.98Sr0.02CuO4, are poorer compared to the GdCo0.95Ni0.05O3, a significant improvement due to a better compatibility delivered by similar electrical and thermal conductivities (Figure 6C) can be observed.

Figure 7.Temperature dependence of the Figure of Merit (ZT) and thermoelectric compatibility factor (s) of the p-type La1.98Sr0.02CuO4 and the n-type CaMn0.98Nb0.02O3 materials.
Summary
Thermoelectric heat-to-electricity conversion has received increasing attention over the past 15 years as one of the ways to improve the energy efficiency of the conventional electric-power production strategies. Thanks to their flexibility, perovskite-oxide materials and derivatives offer a wide range of possibilities to search and optimize novel thermoelectric materials. The advantages of the perovskites are their profound flexibility and relatively simple—and therefore predictable— structure-property relations. On the other hand, the simplicity of the perovskite structure can be seen as a challenge when considering the ways to suppress thermal conductivity. Despite lower performance compared to more conventional thermoelectric materials, such as chalcogenides, the all-oxide materials have their undisputable strengths, such as being able to operate at much higher temperatures and oxidizing atmospheres.
Acknowledgments
The Swiss Federal Office of Energy (SFOE), the Swiss National Science Foundation (SNF-MANEP), and Swisselectric are highly acknowledged for financial support. K. Koumoto, A. Maignan, and J. Hulliger are acknowledged for their fruitful discussions and scientific input. We also gratefully acknowledge the present and former members of the Solid State Chemistry and Catalysis lab at EMPA for their experimental results.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?