Batteries, Supercapacitors & Fuel Cells
Batteries, fuel cells, and supercapacitors are systems using different electrochemical energy storage and conversion mechanisms but similar electrochemical features for high energy and high-power density applications.
Featured Categories
Battery materials ensure reproducible data, supporting research needs from bench-scale to manufacturing for reliable performance.
We offer a wide range of high-purity salts, including nitrates, oxalates, halides, sulfates, carbonates, and acetates, available in both anhydrous and hydrated forms.
We offer an extensive range of high purity oxides and ceramics with specialized synthesis and purification techniques, and distinct particle sizes.
We offer an extensive range of high-purity metals and alloys in diverse compositions and particles size, even customized for your research and production applications.
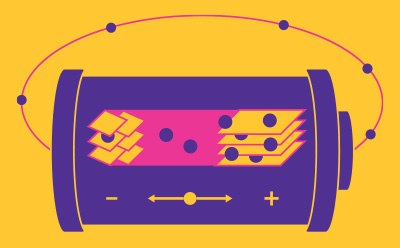
Batteries
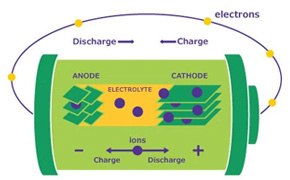
A battery consists of electrodes (cathode (+) and anode (-)), a conductive electrolyte, and a separator between the anode and the cathode. In rechargeable lithium-ion batteries (LIB), monovalent lithium cations migrate between the electrodes. When discharging, the anode (-) oxidizes (loses electrons) and the cathode undergoes reduction (gain of electrons). Upon charging, this process is reversed. Due to their high energy, power density, improved safety and lower material costs, LIBs have revolutionized the electronics industry and are integrated in many aspects of our lives, from mobile devices to electric vehicles. In 2019, the Nobel Prize in Chemistry was awarded to the scientists who developed the LIB technology.
Visit our document search for data sheets, certificates and technical documentation.
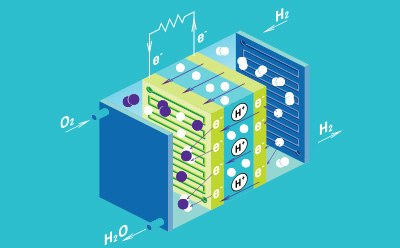
Fuel Cells
Fuel cells consist of an anode, cathode, and a conductive electrolyte, and are often connected in a series to form a stack to increase the total amount of generated electricity. The electrode is comprised of a porous material that is coated with a catalyst to generate electricity. There are five main types of fuel cell types, which are differentiated by the type of electrolyte used: polymer electrolyte membrane, solid oxide, phosphoric acid, alkaline, and molten carbonate. Polymer electrolyte membrane, also known as proton-exchange membrane, (PEM) technology is considered the most promising to replace alkaline fuel-cell technology.
Fuel cells have been developed as an alternative energy technology, due to their high efficiencies, low emissions, and low environmental impact, outcompeting traditional combustion engines. Fuel cells generate only heat and water as waste products, making them a promising candidate for future power sources in a wide variety of applications, including portable devices, stationary devices, and transportation solutions.
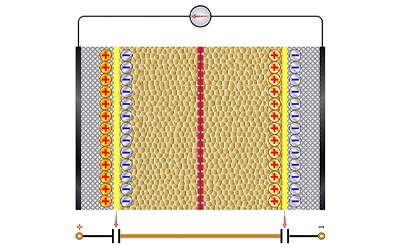
Supercapacitors
The components of supercapacitors are similar to batteries. However, supercapacitors are characterized by their charge storage capabilities. The electrode materials contribute to the storage performance of a supercapacitor and can be divided into three categories: double layer capacitors that act electrostatically, pseudo-capacitors that act electrochemically, and hybrid capacitors that utilize both.
Supercapacitors are a high-density energy source with high energy storage capacity, long shelf life, and quick charging capabilities making them ideal for applications in hybrid vehicles, portable devices, and energy harvesting.
Related Articles
- Professor Aran discusses engineering graphene-based materials through careful functionalization, enabling diverse applications.
- Discover more about advancements being made to improve energy density of lithium ion battery materials.
- Functional materials for printed electronics applications enable flexible displays, RFID tags, and biomedical sensors.
- Recent demand for electric and hybrid vehicles, coupled with a reduction in prices, has caused lithium-ion batteries (LIBs) to become an increasingly popular form of rechargeable battery technology.
- Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures and are composed of two electrodes and a conductive elecrolyte.
- See All (53)
Related Protocols
- Surfactant-assisted dispersion of single-walled carbon nanotubes for debundling or exfoliation in dispersion procedures.
- See All (1)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?