Cancer Stem Cell Detection Utilizing a Red-shifted Fluorescent Live Cell Probe for Aldehyde Dehydrogenase (ALDH), AldeRed™ 588-A.
Introduction
Cancer stem cells (CSCs) are subpopulations of cancer cells that can self-renew, generate diverse cells in the tumor mass, and sustain tumorigenesis. Some researchers believe that cancer arises from cancer stem cells that originate as a result of mutational hits on normal stem cells. High ALDH activity serves as a universal marker of stem cells, both normal and malignant. Cells can be identified and isolated based upon the enzymatic activity of ALDH, a detoxifying enzyme responsible for oxidation of hazardous aldehyde byproducts. The marker ALDH has been used to isolate cancer stem cells from various human malignancies including bladder, breast, cervical, colon, head and neck, liver, lung, pancreas, prostate and ovary. Historically, selection of cells positive for aldehyde dehydrogenase (ALDH) activity within a green fluorescent background has been difficult with existing reagents.
The AldeRed™ ALDH Detection Kit provides cancer and stem cell scientists with new capabilities for live cell isolation and characterization. The AldeRed™ reagent is a red-shifted fluorescent substrate for aldehyde dehydrogenase (ALDH), allowing cancer stem cells to be identified and isolated by flow cytometry with concurrent use of green fluorescent cell lines, antibodies, transgenic animals and reporter assays.
Pomper MG et al. A red-shifted fluorescent substrate for aldehyde dehydrogenase. Nat Commun. 2014 Apr 23;5:3662.
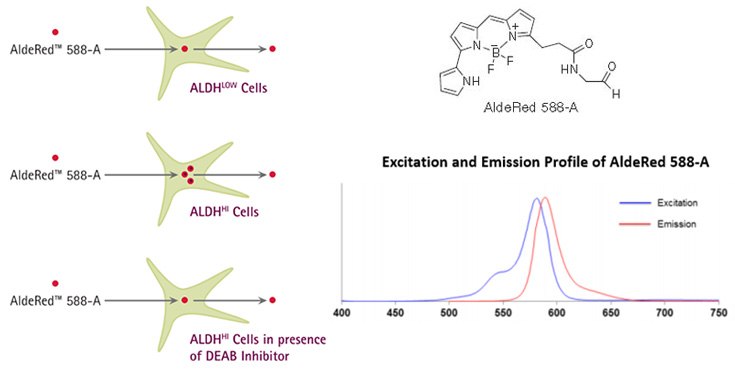
Figure 1. AldeRed™ Mechanism of Action. AldeRed™ 588-A is a fluorescent and non-toxic ALDH substrate that diffuses freely into intact and viable cells, but remains trapped inside the cells once converted by ALDH into the corresponding acid. The amount of fluorescence produced is proportional to the ALDH activity in the cells and is measured by flow cytometry, allowing fluorescence-activated cell sorting (FACS). For optimal excitation and emission of AldeRed™ 588-A, use a combination of blue laser (488 nm) or green laser (532 nm) with PE-Texas Red detector (615 nm).
Methods
Detection of Cancer Stem Cell Populations by Flow Cytometry using AldeRed™ 588–A
A) Reagent Preparation
AldeRed™ Assay Buffer Preparation
- Remove 1 mL of AldeRed™ Assay Buffer from one of the four bottles supplied with the kit and add it to one of four vials containing Verapamil powder. Mix well by vortexing the vial for 2-3 min and transfer the solution back to the same bottle of AldeRed™ Assay Buffer. Label the bottle accordingly.
Important note: AldeRed™ Assay buffer needs to be supplemented with the efflux inhibitor Verapamil to prevent efflux of AldeRed™ 588-A reaction product and loss of fluorescence.
Note: AldeRed™ Assay Buffer supplemented with Verapamil may be stored at 2-8 °C for up to 3 months.
AldeRed™ 588-A Preparation
- Allow all reagents to equilibrate to room temperature.
- Add 25 μL of DMSO to the vial of dry AldeRed™ Reagent and mix well. Expect violet pellet to turn into red color solution upon addition of DMSO.
- Add 25 μL of 2N HCl and mix well. Incubate the mixture for 15 min at room temperature.
- Add 360 μL of AldeRed™ Assay Buffer supplemented with Verapamil to the vial and mix.
- Keep the AldeRed 588-A at 2-8 °C during use.
Note: Aliquot the remaining AldeRed™ 588-A and store at -20 °C in the dark. Avoid repeated freeze thaw cycles.
B) Stem Cell Sample Preparation
- Depending on the cell type you are working with (adherent, suspension, fresh or frozen) follow a standard procedure to suspend cells in growth media and count. Adjust cell number, pellet the cells and replace growth media with 1 mL of AldeRed™ Assay Buffer supplemented with Verapamil.
Note: Cell concentration may require optimization for each cell type (e.g. optimal concentration of SK-BR-3 cells is 1- 2x105 cells/mL, Figure 2). Testing a range of cell concentrations is recommended for determining the highest signal to background ratio (i.e. Test sample to DEAB-treated control sample).
- Label one “test” and one “control” 1.5 mL microcentrifuge tube for each cell sample to be tested. Transfer 1 mL of the cell suspension from Step B to the corresponding “test” tube.
- Add 5 μL of DEAB reagent to each “control” tube. Recap “control” tube and DEAB vial to prevent ethanol evaporation.
- Add 5 μL of the prepared AldeRed™ 588-A from Step A.5 to the “test” tube that contains 1 mL of cell suspension. Mix and immediately transfer 0.5 mL of the mixture to the “control” tube containing DEAB.
- Repeat Steps C.2-3 for each sample to be tested.
- Incubate “test” and “control” tubes at 37 °C for 30-60 minutes. Exact incubation time may require optimization for different cell types.
- Centrifuge all tubes at 250 x g for 5 minutes and discard supernatant. Resuspend cell pellets in 0.5 mL of AldeRed™ Assay Buffer supplemented with Verapamil and keep the cells on ice to prevent ALDH reaction product efflux.
- Optional: Immunophenotyping of intact cells using fluorescent antibodies against cell surface markers may be performed at this point while maintaining the cells in AldeRed™ Assay Buffer supplemented with Verapamil on ice to prevent efflux of AldeRed™ reaction product.
Note: For optimal excitation and emission of AldeRed™ 588-A, use a combination of blue laser (488 nm) or green laser (532 nm) with PE-Texas Red detector (615 nm). Detection in proximal measurement channels (e.g. PE) is also possible, but may affect delta MFI (mean fluorescence intensity) between Test sample and DEAB treated control sample.
- Depending on the make and model of the flow cytometer, the workflow may vary. In set-up mode, adjust FSC and SSC voltages and gains using DEAB “control” sample and create region (R1) to encompass the nucleated cells.
- Create AldeRed™ 588-A vs. SSC dot plot gated on R1 and adjust voltage so the right edge of the DEAB “control” sample cell population is placed at the second decade on X-axis.
- Run a corresponding “test” sample and create a region R2 that contains ALDHHi cells (Figures 2 and 3).
- Switch the flow cytometer to sample acquisition mode and collect data for all “control” and “test” samples.
Results
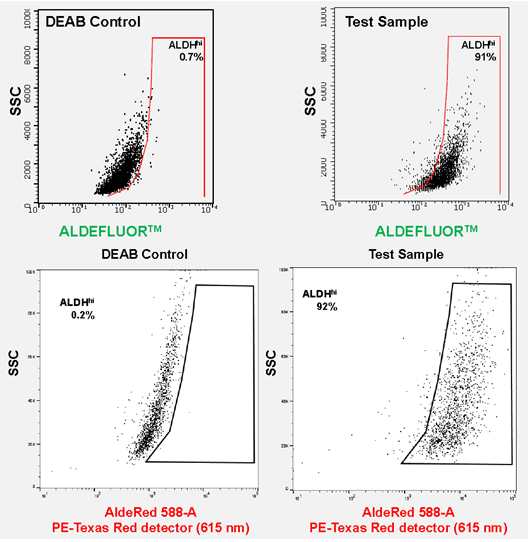
Figure 2. ALDEFLUOR™ and AldeRed™ identify similar CSC populations. ALDH detection in SKBR3 cells using the ALDEFLUOR™ assay yielded 91% ALDHhi population of cells using a combination of Blue laser (488 nm) with FITC detection (525 nm). ALDH detection in SKBR3 cells using the AldeRed™ assay yielded 92% ALDHhi population of cells using a combination of Green laser (532 nm) with PE-Texas red detector (615 nm).
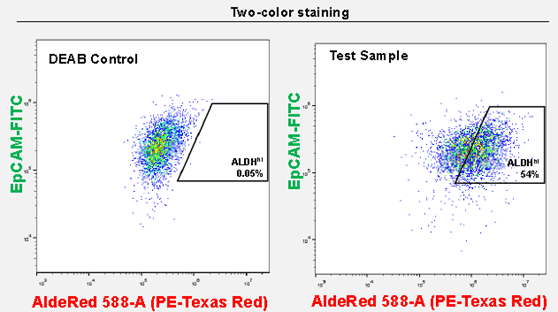
Figure 3. AldeRed™ allows for the co-labeling of CSC populations with FITC-conjugated antibodies. Co-staining of SK-BR-3 cells with AldeRed™ 588-A and Milli-Mark™ EpCam-FITC antibody (clone mAB8 Cat. No. FCMAB264F) yields 54% ALDHhiEpCAMhi.
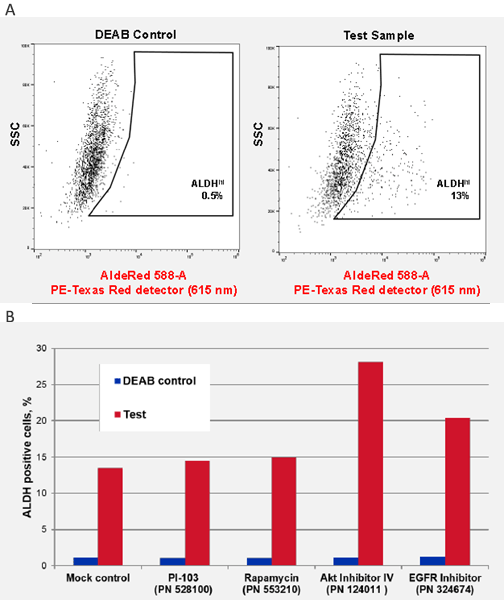
Figure 4. ALDH CSC identification in Head and Neck Squamous Carcinoma cell lines. UM-SCC-47 is a unique head and neck squamous carcinoma cell line isolated from primary tumor and containing integrated human papillomavirus (HPV-16) and characterized by presence of ALDH+ cancer stem cell sub-populations. A) ALDH detection in UM-SCC-47 cancer cell line by AldeRed™ 588-A yields 13% ALDHhi CSC populations. B) Effect of signaling pathway inhibitors on ALDHHi CSC populations. UM-SCC-47 cells were treated for 24 hrs with 1 uM of each inhibitor and ALDH activity was measured by AldeRed™ 588-A. Notably, AKT Inhibitor IV (124011) and EGFR inhibitor (324674) increased the overall ALDH positive CSC populations.
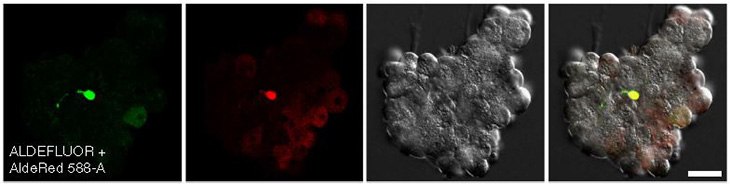
Figure 5. Confocal images of CA/TD murine pancreatic cells co-stained with ALDEFLUOR™ and AldeRed™ 588-A.
Trademarks
ALDEFLUOR™ is a registered trademark of Aldagen Inc.
AldeRed™ is a trademark of Merck KGaA, Darmstadt, Germany and/or its affiliates.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?