How to Fractionate and Analyze Gradients of Percoll
Read more about
- Density Determination Using Density Marker Beads
- Density Marker Bead - Properties
- Effects of Ionic Strength and Sucrose Concentration on Density of Density Marker Beads
- Using Density Marker Beads
- Other Methods for Measuring Density
- Fractionation of Gradients
- Cell Sorting and Counting
- Protein Determination and Enzyme Assay
- Related Materials
Density Determination Using Density Marker Beads
Density Marker Beads are dyed derivatives of Sephadex™. There are ten color-coded bead types, each with a specific density. They have been specifically formulated for use in Percoll gradients and will not work with other media. Using Density Marker Beads as an external marker facilitates monitoring of the gradient shape and range. The position of cells or organelles within the gradient may be accurately located before fractionation using preformed gradients (73,83). The densities of the Density Marker Beads cover the buoyant densities of the vast majority of cells and organelles to be separated in Percoll. In addition to providing a very rapid and simple method for density measurement, using Density Marker Beads provides more accurate data than other methods, since distortion of gradients by fractionation before analysis is completely avoided.
Density Marker Beads are also very useful for standardizing running conditions before carrying out an actual experiment, using the model experiment described previously to generate a series of gradient curves specific for a particular rotor and tube type.
Density Marker Bead - Properties
Each vial contains freeze-dried cross-linked dextran beads having an accurately determined density in Percoll. Nine of the ten bead types can be used for gradients of Percoll containing 0.15 M NaCl or 0.25 M sucrose. Vial 5 is used exclusively for Percoll with 0.15 M NaCl and vial 10 contains beads to be used only for Percoll with 0.25 M sucrose.
The exact density of each type of bead is specific for each manufactured lot, and is printed on the label of each box.
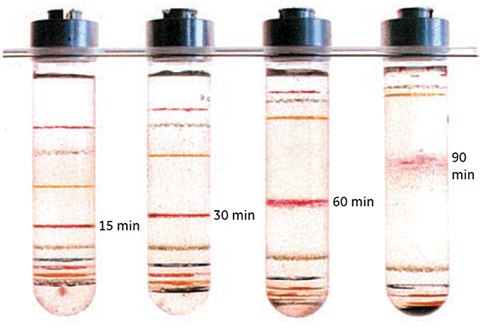
Figure 12.Banding of Density Marker Beads in gradients of Percoll as described in Figure 4 (work from Cytiva, Uppsala, Sweden).
Effects of Ionic Strength and Sucrose Concentration on Density of Density Marker Beads
The densities of Density Marker Beads printed on the label of each box are those recorded under the most widely used conditions (i.e. when Percoll is made isoosmotic with physiological saline or 0.25 M sucrose). The actual buoyant density of the beads will vary slightly with ionic strength or sucrose concentration (osmolality). Figure 13 shows variations of density with ionic strength and Figure 14 shows variations with sucrose concentration. When working with systems outside the normal range of ionic strength or osmolality, these figures may be used as a guideline for calibration of bead densities.
Figure 15 shows the correlation of densities calibrated with Density Marker Beads and by a digital densitometer. This latter method may be used as a crosscheck when working with Percoll in systems outside normal physiological conditions.
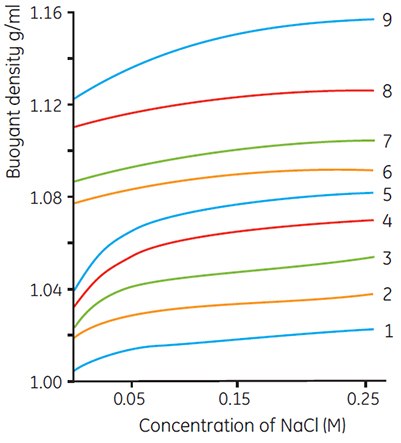
Figure 13.Effects of salt concentration on the recorded densities of Density Marker Beads in gradients of Percoll. Numbers refer to different bead types; the exact density of specific lots is printed on the box label (work from Cytiva, Uppsala, Sweden).
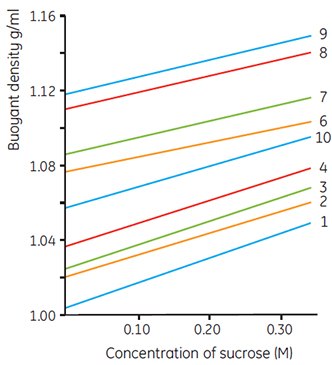
Figure 14.Effects of sucrose concentration on the recorded densities of Density Marker Beads in gradients of Percoll. Numbers refer to different bead types; the exact density of specific lots is printed on the box label (work from Cytiva, Uppsala, Sweden).
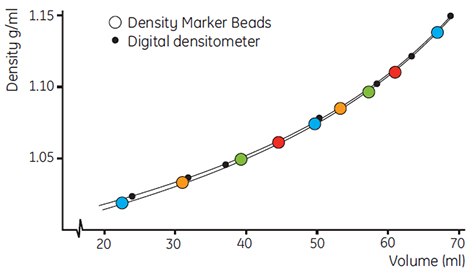
Figure 15.Correlation of recorded densities of a Percoll gradient in 0.15 M NaCl using Density Marker Beads and a digital densitometer (DMA 46, Anton Paar A.G.). Fraction size 2.64 mL, centrifuge MSE Superspeed 75, rotor 10 × 100 mL, angle 18°, 40 000 × g for 60 min (work from Cytiva, Uppsala, Sweden).
Using Density Marker Beads
The beads must be swollen with water prior to use; 1.0 mL of sterile water is added to each vial and the beads are allowed to swell overnight. For long term storage of beads in water, it is advisable to add a preservative such as Merthiolate™ (0.01% w/v).
The quantity of beads required for each experiment will depend on the size of the centrifuge tube, but 10 to 15 μL of suspension is usually sufficient for 10 mL of Percoll. When dispensing the beads with a micropipette, it is useful to snip off the end of the disposable plastic tip to avoid clogging by the beads.
The size of the Density Marker Beads is sufficiently small for them to pass through tubing, monitoring equipment, etc., without problems. Density Marker Beads have been used to monitor gradients of Percoll in zonal centrifuge rotors. Density Marker Beads are used as external markers, in a centrifuge tube containing identical gradient material to the one used for the experiment. They should not be mixed with the cell sample. Density Marker Beads are added to the control tube, which is then used as a counterbalance in the rotor during the centrifugation. The shape of the gradient is measured as described in the model experiment on page 22.
Detailed instructions for use are included in each box of Density Marker Beads.
Note: Density Marker Beads can only be used to calibrate gradients of Percoll. The densities printed on the label do not apply to gradients of other media.
Other methods for measuring density
Several techniques can be used to monitor the density of Percoll solutions after fractionation. Weighing of empty and filled glass micropipettes is accurate but tedious. It is also possible to measure the isopycnic equilibrium point of samples in a precalibrated gradient made from nonaqueous organic liquids (12). Refractive index has a linear correlation with the density of a Percoll solution as shown in Figure 16. Direct measurement using a densitometer (e.g. DMA 3, Anton Paar A.G.) is an accurate alternative to using Density Marker Beads (Fig 15).
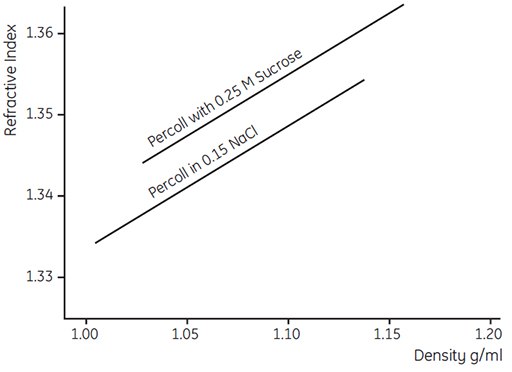
Figure 16.Refractive index as a function of density of a Percoll gradient (work from Cytiva, Uppsala, Sweden).
Fractionation of Gradients
After centrifugation, the gradient can be fractionated by puncturing the bottom of the tube and collecting the outflow into fractions, or by a number of other techniques (1, 28). A simple and convenient method is to collect the fractions from the top of the tube by displacement with a dense medium such as undiluted Percoll, or a 60 to 65% sucrose solution. Upon pumping this dense material to the bottom of the tube, fractions can be drawn off the top. Zonal rotors may be emptied by pumping a denser solution to the distal part of the rotor and collecting fractions from the center.
Cell Sorting and Counting
Percoll does not interfere with fluorescent activated cell sorting (FACS) (911, 1042), or with electronic counting instruments (12). The DNA content of gradient fractions can also be used as a measurement of cell number (12).
Protein Determination and Enzyme Assay
Percoll causes a background color with Folin-Ciocalteau and Lowry reagents, and all measurements should use Percoll solutions for the preparation of the blank. Higher protein concentrations can be determined using the biuret reaction (85). Terland et al. (89) recommend the Coomassie™ Blue method of Bradford (90), since Percoll does not interfere with color development. Vincent and Nadeau (518) have reported a modification of Bradford´s method which involves precipitation of Percoll in a NaOH Triton™ X-100 mixture.
Cell organelles are often identified primarily by the presence of specific enzymes. Many enzyme assays can be carried out in the presence of Percoll without interference. Pertoft and Laurent (21) described an experiment in which the enzymes 5’-nucleotidase (plasma membranes), glucose-6-phosphatase (microsomes), b-glucuronidase (lysosomes) and succinic dehydrogenase (mitochondria) from rat liver homogenates were analyzed in the presence of Percoll. In all cases, the activities were at least as high in Percoll as in the controls indicating that the determinations were not influenced by the medium. Labile succinic dehydrogenase activity was stabilized by Percoll. Aryl sulphatase, alkaline phosphatase, acid phosphatase, b-galactosidase, N-acetyl-a-D-glucosaminidase and b-glucosaminidase have also been analyzed in the presence of Percoll without interference from the medium (21). Due to light scattering by Percoll, it is preferable to use enzyme assays which utilize fluorescence rather than absorbance for detection of activity. For further details of enzyme measurements in Percoll, see references 13, 43, 53, 54, 78 and 89.
To continue reading please sign in or create an account.
Don't Have An Account?