Performing a Separation with Cytiva Products Based on Poly (His) Fusion Proteins
The (His)6 tag is one of the most common tags used to facilitate the purification and detection of recombinant proteins and a range of products for simple, one step purification of (His)6 fusion proteins are available (see Purification options). Polyhistidine tags such as (His)4 or (His)10 are also used. They may provide useful alternatives to (His)6 for improving purification results. For example, since (His)10 binds more strongly to the affinity medium, a higher concentration of eluent (imidazole) can be used during the washing step before elution. This can facilitate the removal of contaminants which may otherwise be co-purified with a (His)6 fusion protein.
Chelating Sepharose, when charged with Ni2+ ions, selectively binds proteins if complex-forming amino acid residues, in particular histidine, are exposed on the protein surface. (His)6 fusion proteins can be easily bound and then eluted with buffers containing imidazole.
Purification and detection of His-tagged proteins, together with information on how to handle fusion proteins when they are expressed as inclusion bodies, are dealt with in depth in The Recombinant Protein Handbook: Protein Amplication and Simple Purification, available from Cytiva.
* Estimate for a (His)6 fusion protein of Mr 27 600, binding capacity varies according to specific protein.
** See Appendix 4 to convert linear flow (cm/h) to volumetric flow rate. Maximum operating flow is calculated from measurement in a packed column with a bed height of 10 cm and i.d. of 5 cm.
Purification Examples
Figures 25 and 26 show the purification of recombinant proteins expressed in soluble form or as inclusion bodies and Figure 27 gives an example of simultaneous on-column purification and refolding of a recombinant protein expressed as an inclusion body.
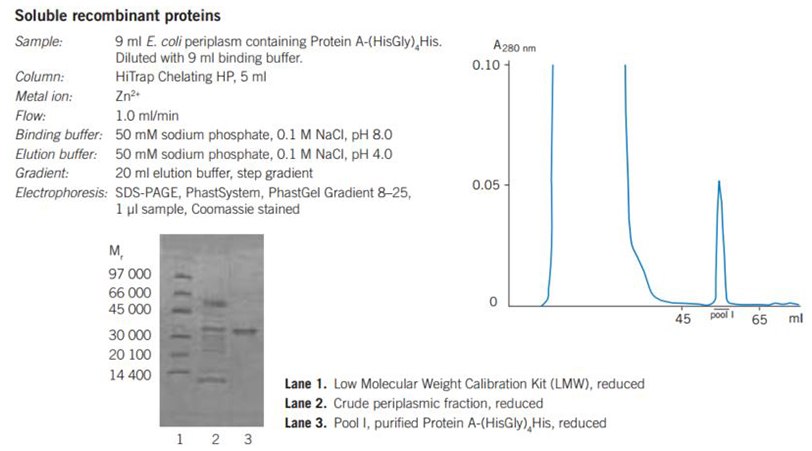
Figure 25. Purification of recombinant proteins on HiTrap® Chelating HP, 5 mL, charged with Zn2+.
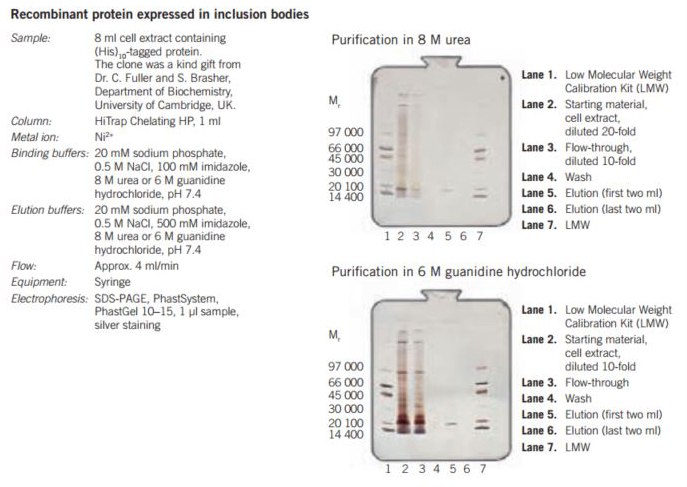
Figure 26. Purification of (His)10-tagged protein from inclusion bodies on HiTrap® Chelating HP, 1 mL, charged with Ni2+.
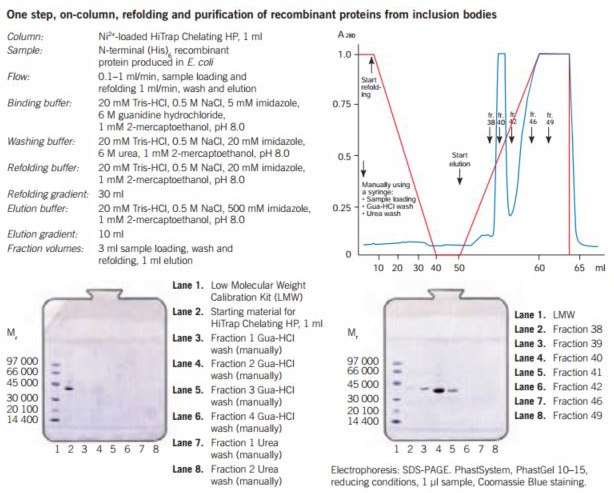
Figure 27. One step refolding and purification of a (His)6-tagged recombinant protein on HiTrap® Chelating HP, 1 mL, charged with Ni2+. The sample is bound to the column and all unbound material is washed through. Refolding of the bound protein is performed by running a linear 6–0 M urea gradient, starting with the wash buffer and finishing with the refolding buffer. A gradient volume of 30 mL or higher and a flow rate of 0.1–1 mL/min can be used. The optimal refolding rate should be determined experimentally for each protein. The refolded recombinant protein is eluted using a 10–20 mL linear gradient starting with refolding buffer and ending with the elution buffer.
Performing a Separation
Figure 28 shows the simplicity of a poly (His) fusion protein purification when using a prepacked HiTrap® Chelating HP column. The protocol described has been optimized for a high yield purification of (His)6 fusion proteins and can be used as a base from which to scale up. An alternative optimization protocol designed to achieve high purity is supplied with the HisTrap® Kit.
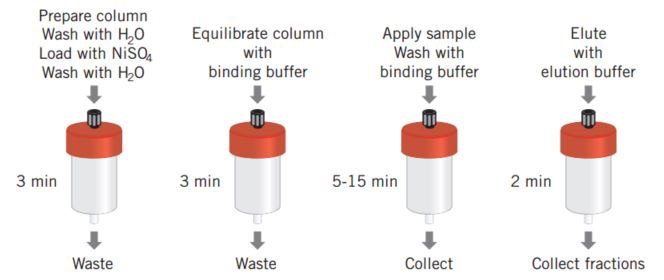
Figure 28. HiTrap® Chelating HP and a schematic overview of poly (His) fusion protein purification.
Nickel solution: 0.1 M NiSO4
Binding buffer: 20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, pH 7.4
Elution buffer: 20 mM sodium phosphate, 0.5 M NaCl, 500 mM imidazole, pH 7.4
- Wash the column with 5 column volumes of distilled water.
Use water, not buffer, to wash away the column storage solution which contains 20% ethanol. This avoids the risk of nickel salt precipitation in the next step. If air is trapped in the column, wash the column with distilled water until the air disappears. - Load 0.5 column volumes of the 0.1 M nickel solution onto the column.
- Wash with 5 column volumes of distilled water.
- Equilibrate the column with 10 column volumes of binding buffer.
- Apply sample at a flow rate 1–4 mL/min (1 mL column) or 5 mL/min (5 mL column). Collect the flow-through fraction. A pump is more suitable for application of sample volumes greater than 15 mL.
- Wash with 10 column volumes of binding buffer. Collect wash fraction.
- Elute with 5 column volumes of elution buffer. Collect eluted fractions in small fractions such as 1 mL to avoid dilution of the eluate.
- Wash with 10 column volumes of binding buffer. The column is now ready for a new purification and there is rarely a need to reload with metal if the same (His)6 fusion protein is to be purified.
Imidazole absorbs at 280 nm. Use elution buffer as blank when monitoring absorbance.
If imidazole needs to be removed, use a desalting column (see page 133, Buffer exchange and desalting for affinity chromatography).
For a single purification of a small quantity of product or for high throughput screening His MicroSpin columns are convenient and simple to use with either centrifugation or MicroPlex 24 Vacuum.
To increase capacity use several HiTrap® Chelating HP columns (1 mL or 5 mL) in series. HiTrap® Chelating HP columns (1 mL or 5 mL) can be used with a syringe, a peristaltic pump or a chromatography system. For even larger capacity, pack Chelating Sepharose® Fast Flow into a suitable column (see Appendix 3, Column packing and preparation).
The loss of metal ions is more pronounced at lower pH. The column does not have to be stripped (i.e. all metal ions removed) between each purification if the same protein is going to be purified. In this case, strip and recharge (i.e. replace metal ions) the column after 5–10 purifications.
Reuse of purification columns depends on the nature of the sample and should only be considered when processing identical samples to avoid cross contamination.
Purification using HisTrap® Kit
HisTrap® Kit includes everything needed for 12 purifications using a syringe. Three ready to use HiTrap® Chelating HP 1 mL columns and ready-made buffer concentrates are supplied with easy-to-follow instructions.
Cleaning
Removal of nickel ions before re-charging or storage:
- Wash with 5 column volumes of 20 mM sodium phosphate, 0.5 M NaCl, 0.05 M EDTA, pH 7.4.
- Wash with 10 column volumes of distilled water.
- For storage, wash with 5 column volumes of 20% ethanol.
Removal of precipitated proteins:
- Fill column with 1 M NaOH and incubate for 2 hours.
- Wash out dissolved proteins with 5 column volumes of water and a buffer at pH 7.0 until the pH of the flow-through reaches pH 7.0.
Chemical Stability
Stable in all commonly used aqueous buffers and denaturants such as 6 M guanidine hydrochloride and 8 M urea.
Storage
Wash media and columns with 20% ethanol at neutral pH (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
The column must be recharged with metal ions after long term storage to reactivate the medium.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?