ARGET ATRP: Procedure for PMMA Polymer Brush Growth
Surface Cleaning and Initiator Immobilization
Surface preparation before polymer brush growth consists of two steps: surface cleaning and initiator monolayer deposition.
There are many common techniques for rigorous surface cleaning; however, the choice is dependent on substrate type, the degree of contamination, and the desired cleanliness of the surface. Heavily contaminated inorganic substrates can be cleaned by washing and ultrasonication in aqueous surfactants and solvents, followed by immersion in piranha solution (H2O + H2O2 + H2SO4 — warning, extremely hazardous). Silica surfaces (including silicon wafers) can be cleaned using the less hazardous RCA-1 clean (H2O + H2O2 + NH4OH) in place of piranha solution, which introduces silanol (Si-OH) groups to the wafer surface to allow reaction with silanes. For less heavily contaminated inorganic surfaces, a ′dry′ clean using UV/O3 exposure will often suffice.
Organic surfaces such as polymers or cellulose are often cleaned simply using solvents. If the material does not intrinsically contain nucleophilic amine or hydroxyl groups, the surface can be ′activated′ by a variety of means (e.g., UV/O3 or O2 plasma to introduce hydroxyl groups, treatment of polyesters with ethylenediamine to introduce amine groups).24
After the surface is clean the initiator monolayer can be formed using a method specific to the surface type.
- Gold and other noble metals surfaces: Monolayers of initiator can be formed by simply immersing the surface in a dilute solution of a disulfide initiator (Product No. 733350) (1-5 mM in ethanol) for 16- 24 hours under ambient conditions, followed by washing with ethanol.
- Organic hydroxyl functional surfaces (e.g., cellulose or activated polymer surfaces): ATRP initiator sites are introduced by reacting surface hydroxyls or amines with 2-bromoisobutyryl bromide (BIBB, Product No. 252271) in anhydrous solvent with an amine base. A typical procedure for cellulose is given below:
- Cellulose (fibers or paper) is placed in a test tube sealed with a septum and the tube deoxygenated by nitrogen purging or vacuum/nitrogen cycling.
- Anhydrous tetrahydrofuran (THF, 10 mL, Product No. 401757) is syringed over the cellulose. BIBB (0.26 mL, 2.10 mmol) and anhydrous triethylamine (0.30 mL, 2.10 mmol) are added by syringe and the tube gently agitated to mix. Note: triethylamine should be dried using 4 Å molecular sieves before use.
- After 1 hour remove the cellulose from the tube, wash with THF, methanol and deionized water and then dry under a nitrogen stream.
3. Silica/silicon and other metal oxides: Many publications use presynthesized silane ATRP initiators for these surfaces. However, a procedure suitable for all levels of synthetic expertise is provided below (and illustrated in Figure 1), using only commercial reagents:
- Clean substrates are placed in a vacuum desiccator or vacuum oven with a vial containing 10 drops of (3-aminopropyl)triethoxysilane (APTES, Product No. A3648). The chamber is then pumped down to <1 mbar, isolated from the pump and left under vacuum for 30 minutes.
- Substrates are then annealed at 110 °C in air at atmospheric pressure for 30 minutes. Note: if using the same vacuum oven for both steps, remove the APTES before heating.
- After annealing, substrates can be reacted directly with BIBB, using the same procedure as for cellulose, above.
Initiator-coated substrates can be stored in air for several weeks without significant loss of activity.

Figure 1. Surface functionalization with silane ATRP initiator.
Polymer Brush Growth by ARGET ATRP
A typical procedure for the growth of PMMA polymer brushes is given below, and illustrated in Figure 2.
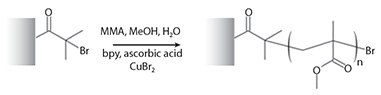
Figure 2. Polymerization of methyl methacrylate from a surface.
Samples can be handled using stringent inert atmosphere with the polymerization taking place in a deoxygenated tube or under less stringent conditions in a screw-top jar (Figure 3). Several ARGET ATRP brush growth polymerization methods will work adequately under the less stringent conditions, which also greatly simplifies handling. Under less stringent conditions fresh samples can simply be dipped into the solution and removed when desired, although the jar should be sealed for the majority of the polymerization time.
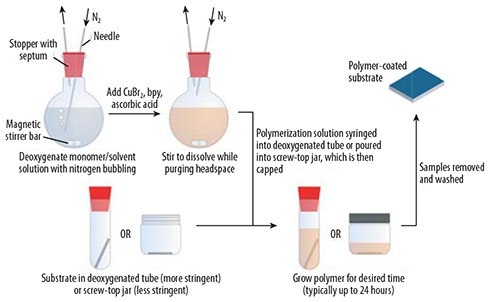
Figure 3. Typical experimental procedure for SIP by ARGET ATRP.
- Initiator-coated substrates are placed in test tubes which are deoxygenated by nitrogen purging or vacuum/nitrogen cycling. Alternatively, they can be placed in a screw-top jar under air.
- In a round-bottomed flask sealed with a septum, methanol (16 mL), water (4 mL) and methyl methacrylate (20 mL, 18.72 g, 187.0 mmol, Prod. No. M55909) are mixed and deoxygenated by bubbling through nitrogen for 10-15 minutes.
- CuBr2 (7.4 mg, 0.033 mmol, Prod. No. 221775), 2,2′-dipyridyl (bpy, 51.5 mg, 0.33 mmol, Prod. No. D216305) and sodium L-ascorbate (65.3 mg, 0.33 mmol, Prod. No. A7631) or ascorbic acid (58.1 mg, 0.33 mmol, Prod. No. A0278) are added and the headspace purged with nitrogen. The mixture is stirred to dissolve the solids.
- The solution is syringed over the substrates in the deoxygenated tubes, or simply poured over the substrates in the screw-top jar, which is then resealed. The samples are allowed to polymerize at ambient temperature.
- After the desired polymerization time, samples are removed and washed with methanol and water. If free polymer is observed on the samples, this can be removed by washing with THF or other appropriate solvents for PMMA — the polymer coating will not be damaged by solvent washing.
Typically, this method produces a polymer growth rate of approximately 10 nm/hour (Figure 4).
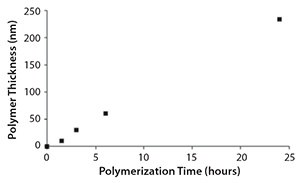
Figure 4. PMMA Polymer brush thickness during polymerization under stringent deoxygenation conditions at 20 °C (measured by ellipsometry).
Materials List
如要继续阅读,请登录或创建帐户。
暂无帐户?