Cross-Coupling Reactions in Water
Description
TPGS-750-M, DL-α-Tocopherol methoxypolyethylene glycol succinate, is a designer surfactant composed of a lipophilic α-tocopherol moiety and a hydrophilic PEG-750-M chain, joined by an inexpensive succinic acid linker. TPGS-750-M spontaneously forms micelles upon dissolution in water. The balance and composition of lipophilic and hydrophilic components have been tailored to promote a broad array of chemistry in water. Furthermore, this new, more practical surfactant can be readily be substituted for older amphiphiles, usually with equal or greater efficiency in terms of both yield and reaction times.
Conducting transition metal-catalyzed cross-coupling chemistry in water instead of organic solvent has a number of potential benefits in terms of cost, environmental impact, safety, and impurity profiles. Increasing focus on “Green” chemical processes has further promoted recent developments in this field. The actual means of implementing reactions in water, however, especially at room temperature and for water insoluble, organic substrates, has not always been clear.
One solution applied to a broad range of transition-metal-catalyzed processes is the use of small amounts of a nanomicelle-forming amphiphile in water, which provides a lipophilic medium where cross-coupling reactions can take place. Initially, the viability of surfactant promoted, transition metal-catalyzed chemistry in water at room temperature was investigated with a variety of surfactants with PTS, an α-tocopherol based diester of sebacic acid, the most effective. A number of palladium and ruthenium-catalyzed processes were found to be amenable to mild, room temperature reactions in water. Products can be recovered from the aqueous phase using standard extraction procedures and in high isolated yields.1 TPGS-750-M is a second generation surfactant designed with physical properties to promote a broader array of chemistry in water more efficiently than seen with PTS.2
Precautions
Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Procedures
Heck Reaction

The catalyst Pd[P(t-Bu)3]2 (5.1 mg, 0.01 mmol, Catalog Number 676578) and aryl iodide (0.50 mmol) are added under argon into a reaction flask containing a stir bar. An aliquot of TPGS-750-M/H2O solution (1.0 mL; 5 wt % TPGS-750-M in water, Catalog Number 763918), Et3N (0.21 mL, 1.50 mmol), and acrylate/styrene (1.0 mmol) are added sequentially by syringe, and the resulting mixture is allowed to stir at room temperature for 4–12 h. The homogeneous reaction mixture is then diluted with EtOAc (2 mL) and filtered through a bed of silica gel, and the bed is further washed (3 × 5 mL) with EtOAc to collect all of the coupled material. The volatiles are removed in vacuo to afford the crude product, which is subsequently purified by flash chromatography on silica gel.
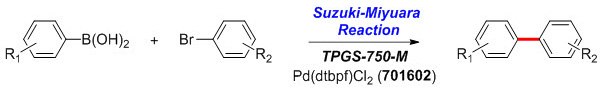
Arylboronic acid (0.50–1.00 mmol), aryl bromide (0.50 mmol), and Pd(dtbpf)Cl2 (6 mg, 0.01 mmol, Catalog Number 701602) are added to a reaction flask containing a stir bar. Under a positive flow of argon while stirring, an aliquot of TPGS-750-M/H2O solution (1.0 mL, 2 wt % TPGS-750-M in water, Catalog Number 733857), and Et3N (0.21 mL, 1.50 mmol) are added by syringe and stirred vigorously for 2–24 h. The reaction mixture is then diluted with brine and extracted with EtOAc. The solution obtained is dried over anhydrous MgSO4 and concentrated by rotary evaporation, which is subsequently purified by flash chromatography on silica gel.

The catalyst Pd(CH3CN)2Cl2 (1.3 mg, 0.005 mmol, Catalog Number 446319) and XPhos (6.2 mg, 0.013 mmol, Catalog Number 638064) are added under argon into a reaction flask containing a stir bar. An aliquot of TPGS-750-M/H2O solution (1.0 mL; 3 wt % TPGS-750-M in water, can use Catalog Number 763896 and dilute in H2O), Et3N (0.14 mL, 1.00 mmol), aryl bromide (0.50 mmol), and alkyne (0.75 mmol) are added sequentially by syringe, and the resulting solution is allowed to stir at room temperature for 21–25 h. The homogeneous reaction mixture is then diluted with EtOAc (2mL), and filtered through a bed of silica gel, and the bed is further washed (3 × 5 mL) with EtOAc to collect all of the coupled material. The volatiles are removed in vacuo to afford the crude product, which is subsequently purified by flash chromatography on silica gel.

The catalyst [(p-allyl)PdCl]2 (2.1 mg, 0.006 mmol, Catalog Number 222380), ligand cBRIDP (7.6 mg, 0.022 mmol, Catalog Number 693383), KO-t-Bu (184 mg, 1.56 mmol), and amine (1.20 mmol) are sequentially added under argon into a reaction flask containing a stir bar. An aliquot of TPGS-750-M/H2O (1.0 mL; 2 wt % TPGS-750-M in water, Catalog Number 733857) solution and aryl bromide (1.00 mmol) are added by syringe, and the resulting solution is allowed to stir at room temperature for 19–20 h. The homogeneous reaction mixture is then diluted with EtOAc (2 mL) and filtered through a bed of silica gel, and the bed is further washed
(3 × 5 mL) with EtOAc to collect all of the coupled material. The volatiles are removed in vacuo to afford the crude product that is subsequently purified by flash chromatography on silica gel.

In a reaction flask under argon containing zinc dust/powder (195 mg, 3 mmol) and PdCl2(Amphos)2 (3.5 mg, 0.005 mmol, Catalog Number 678740) is added a solution of 2 wt % of TPGS-750-M in water (3.0 mL, Catalog Number 733857).
N,N,N′,N′-tetramethylethylenediamine (116 mg, 1 mmol, Catalog Number 411019) is added at room temperature followed by the addition of alkyl halide (2.0–3.0 mmol) and aryl or alkenyl bromide (1 mmol). The flask is stirred vigorously at room temperature for 12–48 h. The reaction mixture is then filtered through a bed of silica gel and the bed is further washed (3 × 5 mL) with Et2O to collect all of the coupled material. The volatiles are removed in vacuo to afford the crude product that is subsequently purified by flash chromatography on silica gel.

Alkene (0.50 mmol), acrylate (1.00 mmol)/ketone (1.50 mmol), and Grubbs-2 catalyst (8.5 mg, 0.010 mmol, Catalog Number 569747) are sequentially added into a reaction flask containing a stir bar. An aliquot of TPGS-750-M/H2O (1.0 mL; 2.5 wt % TPGS-750-M in water, can use Catalog Number 763896 and dilute in H2O) is added via syringe, and the resulting solution is allowed to stir at room temperature for 12 h. The homogeneous reaction mixture is then diluted with EtOAc (2 mL) and filtered through a bed of silica gel, and the bed is further washed (3 × 5 mL) with EtOAc to collect all of the cross-coupled material. The volatiles are removed in vacuo to afford the crude product that is subsequently purified by flash chromatography on silica gel.
References
To continue reading please sign in or create an account.
Don't Have An Account?