Alzheimer’s In A Dish™: 3D Neural Stem Cell Models of Alzheimer's Disease
Section Overview
- Introduction
- Protocol Used to Generate 3D NSC Models of Alzheimer’s Disease
- Human ReNcell® VM NSC Maintenance and Passaging
- Viral Infection of Human ReNcell® VM NSCs
- Enrichment of High-Expressing Transgenic Human ReNcell® VM NSCs
- Culturing Sorted Human ReNcell® VM NSCs
- 3D Matrix Cultures of Human ReNcell® VM NSCs
- 3D Culture Cell Seeding of Human ReNcell® VM NSCs
- Results
- Related Materials
Introduction
3D cell culture technologies aim to provide improved predictive cellular models for research, drug discovery or regenerative medicine applications. Historically, In vitro human cell models of Alzheimer’s disease (AD) have been challenging due to high levels of soluble and insoluble toxic amyloid β (Aβ) species that do not recapitulate the true AD pathology. Recently, Kim et. al created a three-dimensional (3D) human neural stem cell model of Alzheimer’s Disease using β-amyloid precursor protein and presenilin-1 overexpressing ReNcell™ VM human neural stem cell lines. This 3D cell model was able to induce robust extracellular deposition of amyloid-β, including amyloid-β plaques, and high levels of phosphorylated tau in the soma and neurites, as well as filamentous tau. This model is a valuable tool to study age-related AD dementia.1,2,3
ReNcell lines are highly published human neural stem cell lines derived from developing human brains. ReNcell® VM and CX cells are generated from the ventral mesencephalon and cortical regions of the brain, respectively, and transduced with the myc transcription factor. Both cell lines offer phenotype and genotype stability, in addition to the multipotential neuronal and glial differentiation capacity over long-term culture.
Protocol Used to Generate 3D NSC Models of Alzheimer’s Disease
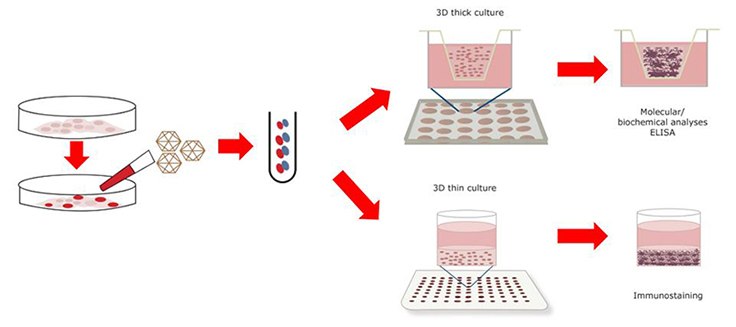
Human ReNcell® VM NSC Maintenance and Passaging
- Warm the ReNcell® NSC Maintenance Media and Accutase solution in a 37 °C water bath for at least 10 min.
- Wash ~95% confluent ReNcell® VM cells in a T25 flask with 3 mL of D-PBS, aspirate the D-PBS and add 0.5 mL of Accutase under a biosafety culture hood.
- Incubate the cells in a 37 °C CO2 incubator for 3–5 min, add 3 mL of prewarmed ReNcell® NSC Maintenance Media and transfer the cell suspensions to a 15-mL sterile conical tube.
- Spin down the cells at 2,000g for 3 min at room temperature, remove the supernatant and resuspend the cell pellet in 12 mL of ReNcell® NSC Maintenance Media.
- Dispense 4 mL of the ReNcell® suspension into each Matrigel-coated T25 flask, and then incubate it for 3–4d in a 37 °C CO2 incubator.
Note: After splitting (1:3 ratio), ReNcell® cells generally grow confluent after 3–4d. If cells are not confluent at that time, replace the medium with fresh ReNcell® NSC Maintenance Media and wait for 1 or 2d.
Viral Infection of Human ReNcell® VM NSCs
- To prepare ReNcell® VM cells for infection, dislodge and spin down the cells from a confluent T25 flask, as described in Steps 1–4. Resuspend the cell pellet in 12 mL of prewarmed ReNcell® NSC Maintenance Media and dispense 2 mL of the cell suspension into each well of an Matrigel-coated six-well plate. Gently cross-shake the dish, and allow the cells to settle overnight.
- Replace the medium with 2 mL of prewarmed ReNcell® NSC Maintenance Media. When cells reach 70–80% confluence, add 6 × 106 transducing units (TU) of viral particles per well to achieve an approximate multiplicity of infection (MOI) of 1. Mix gently and incubate the cells overnight.
- Wash the cells twice with 2 mL of prewarmed ReNcell® NSC Maintenance Media, and then apply 2 mL of the medium. Confluence should increase over time. Transgene expression can be expected 48 h after infection and should be detectable by fluorescence microscopy.
Note: Carefully monitor the culture. If abnormal cell death is observed, replace the medium immediately.
- On day 4, if the cells are confluent, passage the cells as described in Steps 1–5 and recommence normal culturing. If the cells did not reach complete confluence by this point, replace the medium instead of passaging, and incubate until the cells reach confluence. To generate cells expressing both APPSL/GFP and PSEN1(E9)/mCherry (ReN-mGAP cells), the transduced cells can be infected again with different lentiviral vectors after splitting, as described in Steps 6–8.
- Validate the infection by microscopic detection of GFP and mCherry fluorescence and western blot analysis of overexpressed APPSL and PSEN1(E9). Typically, >50% of infected cells visibly express the fluorescent marker.
Note: The infected, validated cells can be frozen and stored in a liquid LN2 tank at least for 1 year before flow cytometry analyses.

Figure 1. Fluorescence images of ReNcell VM infected with APPSL-GFP (A) or PSEN1-RFP (B) Lentivirus, MOI =20. By day 3 post viral transduction >80% ReNcell VM express the Alzheimer’s associated transgenes.
Enrichment of high-expressing transgenic Human ReNcell® VM NSCs
- Culture the transduced ReNcell®hVM cells until confluence is reached (a 95% confluent T25 flask yields ~2–3 × 106 cells). Although the optimal total cell count depends on the infection efficiency, at least 2–3 × 106 successfully transduced cells should be available (when assayed by fluorescent cell sorting or fluorescence microscopy).
- Detach the cells as described in Steps 1–4, but resuspend the cell pellets with 4 mL of D-PBS. Determine the cell number using an automated cell counter.
- Spin down the cells briefly at 2,000g at room temperature, remove the supernatant and resuspend the cell pellets in ice-cold sorting medium (1 mL per 107 cells).
- To singularize the cells, aspirate the cell suspension with a 1000uL pipette, gently press the tip against a cell strainer mesh at a 90° angle and empty the pipette forcefully. In case of blockage, carefully move the pipette tip across the mesh while maintaining pressure. Collect the filtered suspension in a 15-mL centrifugation tube.
Note: The suspended cells can be stored on ice at least for 1 h before fluorescence-activated cell sorting.
- Perform fluorescence-activated cell sorting using GFP (488 nm, 200 mW excitation, 530/30 emission) and mCherry (561 nm, 150 mW excitation, 610/20 emission) to define and sort populations for the final bulk sorting. Set gates to select cells with high expression of APPSL/GFP alone, APPSL/PSEN1(E9)/mCherry alone or APPSL/GFP and PSEN1(E9)/mCherry together.
- Immediately after sorting, plate the cells into Matrigel matrix-coated dishes.
Note: The suspended cells can be stored in a cell collection tube on ice for up to 1 h before plating.
Culturing Sorted Human ReNcell® VM NSCs
- Spin the cells down at 2,000g for 3 min at 4 °C, and then remove the supernatant and resuspend the pellet in prewarmed ReNcell® NSC Maintenance Media (1 mL per 2 × 106 collected cells).
Note: Determine the cell concentration after resuspension and adjust the volume accordingly. Even counts from sorting can lead to substantial overestimation.
- Seed the cells on Matrigel matrix-coated 24-well plates at an initial density of 2 × 105 cells per cm2. Expand the cells serially into Matrigel matrix-coated six-well plates, and finally into Matrigel matrix-coated T25 flasks. Make multiple cell stocks at this stage. Passage the cells as described in Steps 1–5. When sufficient cells have been grown, proceed to the next step.


Figure 2. Alzheimer’s In a Dish™ clonal FAD ReNcell® VM human neural stem cell lines.Alzheimer’s in a Dish™ is a proprietary collection of immortalized single cell derived ReNcell® VM human neural progenitor cells that express stable levels of fluorescently tagged AD genes with multiple FAD mutations. The clonal FAD neural progenitor cells secrete different amounts of total Aβ and differ in the Aβ42/40 ratios. All clonal FAD cell lines express neural stem cell markers Nestin (B) and Sox-2 (C). For example: the ReN-mGAP10 Clone D4 neural progenitor cells (SCC008FAD2) were double infected with polycistronic lentiviruses expressing APP (Swe/Lon)-GFP, (D) PS1 (deltaE9)-mCherry (E), merged images (F).
3D Matrix Cultures of Human ReNcell® VM NSCs
- Take out an Matrigel matrix stock from the −80 °C freezer and place it in a 4 °C refrigerator 1 d before use.
- Grow ~95% confluent control and FAD ReNcell® VM cells in five Matrigel-coated T25 flasks. An approximate number of ReNcell® cells in a confluent T25 flask is 3–4 × 106. It takes generally 2d after passaging the cells.
- Spray down an ice bucket with ethanol and expose it to UV radiation in a cell culture hood for ~20 min.
- Place the ReNcell® differentiation medium and Matrigel stock on ice.
Note: Matrigel matrix tends to solidify at >10 °C. Thaw it at 4 °C overnight and then keep it on ice until use.
- Remove the medium from the T25 flasks with aspirating pipettes. Be careful not to touch the cells with the pipettes; always use the vacuum on the non-cell side.
- Wash the cells once with 3 mL of D-PBS per T25 flask, and then carefully remove it with the aspirating pipettes.
- Add 0.5 mL of Accutase™ reagent into each flask, and incubate the cells at 37 °C for 3–5 min.
- Dislodge the cells by forcefully tapping the side of the flask until clumps of cells completely detach.
- Resuspend the cells with 3 mL of ReNcell® differentiation medium and pipette it up and down inside the flask at least three times.
- Transfer the cell suspensions from all five T25 flasks into a 15-mL tube.
- Centrifuge the tube for 2 min at 2,000g at room temperature.
- Remove the medium with aspirating pipettes.
- Resuspend the cell pellet in 2 mL of cold ReNcell® differentiation medium and vortex for 10s. Set the 15-mL tubes on ice.
- Take a small aliquot of suspended cells and dilute 1:10 for cell counting (10uL of suspension: 90uL of differentiation medium).
- Count the cells using a cell counter slide; dilute the cells if needed (optimum concentration is ~2 × 107 cells per mL before adding Matrigel matrix at a 1:1 ratio in the next steps).
Note: It is desirable to achieve a high cell concentration at this stage. We have found that 1 × 107 cells per mL (after adding Matrigel matrix at 1:1 ratio; see the Step 34) show robust APP and p-tau accumulation in biochemical analysis.
3D culture cell seeding of Human ReNcell® VM NSCs
- Follow option A to make thick-layer 3D cultures or option B for thin-layer 3D cultures.
Note: Plating a high number of cells is important in order to achieve APP aggregates early. The desired number of cells is 1 × 107 cells per mL in an Matrigel matrix mixture. Therefore, enough cells need to be grown at this stage. The passage number and the condition of the cells are also important.
(A) Thick-layer 3D culture (3–4-mm thickness)
- Add cold Matrigel matrix to the cell suspension on ice (1:1 (vol/vol) dilution). Make sure to chill the pipette tips first by pipetting cold differentiation medium back and forth before transferring Matrigel matrix.
- Vortex the mixture for 30 s.
- Dispense 300 μL of the Matrigel/cell suspension mixture into each tissue culture insert in 24-well plates using prechilled pipettes.
- Incubate the plates at 37 °C in a CO2 incubator overnight.
- Add prewarmed (in 37 °C water bath) ReNcell differentiation medium to the plates (1 mL: 500 μL into the insert and 500 μL into the surrounding well) and place them back in the incubator.
- Change half of the volume of the medium every 3–4 d; at the beginning, medium changing may be needed every 2d depending on the medium color. Never leave differentiated cells drying with no medium.
- Differentiate 3D-plated cells for 4–17 weeks, depending on the experiments. Drug treatments should be done in the last 2 weeks before endpoint analyses.
Note: As it is not easy to monitor the thick-layer culture under an optical microscope, it is important to perform a routine LDH release assay to check the status of the cultures. It is also strongly recommended to set up thin-layer 3D cultures from the same Matrigel/cell mixture to monitor the culture quality.
(B) Thin-layer 3D cultures (100–300-mm thickness)
- Add cold Matrigel matrix to the cell suspension on ice (1:1 (vol/vol) dilution). Make sure to chill the pipette tips first by pipetting cold differentiation medium back and forth before transferring Matrigel matrix. For 96-well-plate thin-layer cultures, further dilute 1:1 Matrigel matrix/cell mixture by adding five volumes of the cold ReNcell differentiation medium (1:11 dilution final), and vortex it for 30 s. The same dilution rate can be used for thin-layer 3D cultures in 8-well/16-well chambered cover-glass slides or MatTek™ glass-bottomed dishes.
- Plate 100 μL of the Matrigel matrix/cell suspension mixture per well of a 96-well plate using prechilled pipettes. If a thicker 3D culture is desired, use two drops per well (160 μL using a multichannel pipette). A volume of 200 μL is recommended for eight-well chambered cover-glass slides and 300 μL for glass-bottomed dishes.
- Incubate the plates at 37 °C in a CO2 incubator overnight.
- The next day, add two drops of prewarmed ReNcell® differentiation medium to each well of the 96-well plates (160 μL using a multichannel pipette), 200 μL for eight-well chambered cover-glass slides and 300 μL for glass-bottomed dishes.
- Change half of the volume of the medium every 3–4d; at the beginning, medium changing may be needed every 2 depending on the medium color. Never leave differentiated cells drying with no medium. Drug treatments should be done in the last 2 weeks before endpoint analyses.
Note: ReNcell® differentiation in thin-layer 3D cultures can be closely monitored by optical and fluorescence microscopy. Some of the cultures can be fixed with 4% paraformaldehyde at 2–4 weeks and tested for neural marker expressions using Anti-β-Amyloid antibody (MAB348) and Anti-Phospho Tau (AB9668) by immunofluorescent staining or immunohistochemistry.
Results
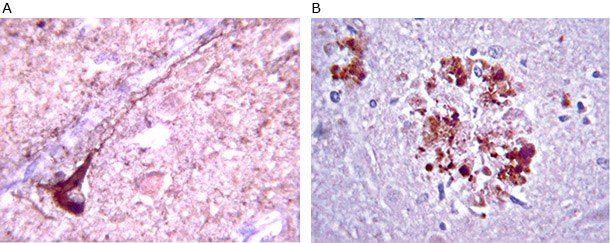
Figure 3. A) Anti-β-Amyloid antibody staining (MAB348) staining on Alzheimer’s diseased cells. Immunoreactivity is seen as staining on plaque deposits (dark brown). B) Anti-Phospho Tau staining (AB9668) of Alzheimer’s diseased cells. Immunoreactivity is clearly not nuclear and it follows the length of the neuron’s axon.

Figure 4. Analysis of amyloid aggregates in 3D culture by Congo red staining. Clonal FAD ReNcell NSCs (SCC008FAD2, SCC008FAD3, SCC008FAD5) were 3D differentiated for 7 weeks and extracted with mild detergent. The pellet (insoluble) fractions were resuspended in PBS, loaded and fixed in glass slides and stained with 1% Congo red solution (HT60).
Cell Lines
Lentiviral Particles
Cell Media and Reagents
Antibodies and Stains
References
To continue reading please sign in or create an account.
Don't Have An Account?