Higher cell densities and antibody titers from increasingly advanced upstream processes result in complex downstream purification challenges. The focus of downstream bioprocessing is efficient recovery and purification while controlling bioburden and assuring viral safety to provide drug safety for patients. The downstream workflow, which includes clarification, chromatography, concentration, viral clearance, and sterile filtration steps, is essential for ensuring monoclonal antibody (mAb) product quality, yield, safety, and sterility.
Virus Filtration
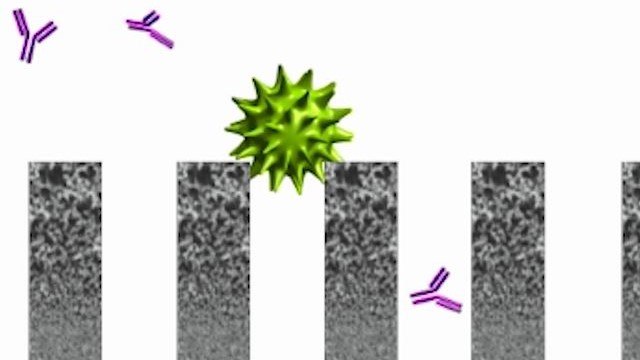
To ensure the safety of monoclonal antibody (mAb) therapeutics, downstream purification steps must effectively clear viruses of different physicochemical characteristics. Virus filtration is a dedicated viral clearance step used in most production processes that provides size-based virus removal and complements other virus removal technologies such as chromatography or viral inactivation through chemical treatment. Understanding the parameters that impact the virus filtration performance is essential to optimize virus filter capacity, flux, and retention.
Our virus filtration portfolio comprises:
- Bioreliance® Contract Testing Services Viral Clearance Testing
- Millipore® Virus Filters
- Millipore® Virus Filter Holders and Single-Use Virus Filtration Systems
- Millipore® Automated Integrity Tester
- Millipore® Services (single-use and filter validation, equipment installation, system qualification, system training, system services)
- Millipore® Automation and Analytics Software
Related Products
Related Categories
Our broad portfolio of buffer materials manufactured under appropriate controls is tailored to your needs.
Clarification steps prepare your bioreactor harvest material for downstream purification by reducing the levels of impurities and particulates.
Downstream purification in bioprocessing includes one or more chromatographic separation steps to purify your molecule and remove impurities.
Our broad portfolio of membrane filters offers options for efficient processing of every pharma and biopharma stream.
Our viral clearance portfolio includes the virus filtration solutions, chemicals, holders and systems.
Ultrafiltration & diafiltration increase capacity, concentration, and recovery in bioprocessing.
Related Resources
- Viral Safety in Bioprocessing
This article discusses viral safety strategies and invites you to download our Viral Safety eHandbook for more insights.
- Application Note: How Baseline Studies Can Ensure Successful Virus Filtration Spiking Studies
Demonstrating the viral clearance capabilities of specific unit operations in downstream processing is a key component of regulatory submissions for biopharmaceuticals. Understanding mitigation actions to assure representative performance is key to successful filtration clearance studies using the Viresolve® Pro Device.
- Application Note: Virus Retention Performance of Viresolve® Pro Devices under a Range of Processing Conditions
This document outlines virus retention performance of Viresolve® Pro Device, using a Quality by Design (QbD) approach. These data help biologics manufacturers understand the factors that affect device performance.
- Application Note: Multi-tier Approach to Assuring Virus Retention and Integrity of Viresolve® Pro Devices
Virus retentive filters are routinely used in biologics manufacturing to ensure a robust, effective virus clearance. This document summarizes our multi-tier approach to assure retentive performance and integrity of each Viresolve® Pro Device.
- Article: Virus Filtration Process Development Optimization
The Key to a more efficient and cost-effective step.
- Guide: Viresolve® Pro Solution. Prefilter Selection Guide
Prefilters increase the capacity of virus filters and improve process robustness. This guide summarizes key considerations when selecting a virus prefilter.
- Flyer: Viresolve® Pro Solution: Robust. Productive, Proven
The Viresolve® Pro Solution is tailored to meet specific needs, offering robust, productive, and proven virus filtration performance. It leads the market with exceptional retention assurance and productivity. Key features include high flux and capacity, consistently high virus retention, easy scalability, usage, and integrity testing. Additionally, it provides multilevel quality assurance and enhances process economics.
Explore Our Solutions
To continue reading please sign in or create an account.
Don't Have An Account?