High density receptor-ligand binding assays
Abstract
The MultiScreenHTS 384-well filter plate is used to perform receptor-ligand binding assays using the Human muscarinic M1 G-protein Coupled Receptor (GPCR) as a representative model system. The data presented demonstrates that both quantitative and screening (displacement) type assays can be performed with the same robustness and reproducibility as a 96-well filter plate format. Receptor ligand binding in the 384-well filter plate can be performed with half the reagents in half the assay volume as the 96-well format, offering significant cost savings per sample. In addition, receptor-ligand binding assays can be performed directly in the new MultiScreenHTS 384-well filter plate decreasing the number of manipulations, and reducing radioactive solid waste by eliminating the need for a separate incubation plate.
Background
Receptors are biological macromolecules capable of highly specific binding to chemical ligands. They are located either in the cell membrane, the cytoplasm or the cell nucleus. Upon binding of a receptor by a ligand, signal transduction events occur which regulate various biological processes required for growth and function of the cell. A great many disease states can be treated by regulating the activity of receptors and/or their downstream effector molecules. For instance, uncontrolled cellular proliferation during cancer can be reduced by blocking the activity of growth receptors. In addition to blocking the activity of receptors, many diseases can be treated by replacing the activity of an endogenous ligand with a more potent chemical entity, as in the case when either the production of the ligand is limited or changes in the receptor alter its specificity for the natural ligand. Thus, a major focus in drug discovery involves identifying compounds which bind specifically to cellular receptors to reduce (antagonists) or augment (agonists) a biological process.
A particular class of receptor that is involved in a large number of disease states is the G-Protein Coupled Receptor (GPCR). GPCRs comprise the largest family of transmembrane receptors in the human genome. Due to the transmembrane structure of the GPCR class of receptors, which make them integrally embedded in the cell membrane1, these receptors are difficult to purify from the cellular fraction in an active form. Purification from the cellular membrane often requires extensive optimization to maintain activity. Identification of
key ligand binding regions which can be dissected away from the entire polypeptide may be needed to perform binding studies. For these reasons, techniques which can characterize binding of intact GPCRs while they are present in the cellular membrane are highly desirable.
Heterogeneous filter binding assays are an established reference method (particularly under more challenging conditions, e.g. using unpurified tissue homogenates or cell membrane fragments) to study receptor-ligand binding. To increase the assay throughput, these heterogeneous binding assays have been successfully adapted to the 96-well filter plate platform2. However, an increasing number of compounds available from natural product isolation and combinatorial chemistry has created a bottleneck making it necessary to develop higher throughput platforms that make it possible to perform adequate library screening in a timely and cost effective manner.
The 384-well filter binding format presented here fulfills these needs by reducing the amount of reagents for each assay, and by performing and quantifying the binding reactions directly in the same 384-well plate.
Introduction
The MultiScreenHTS 384-well filter plate has been developed and optimized for automated radiometric receptor-ligand binding assays. This 384-well filter binding format increases throughput of receptor-ligand binding screening without sacrificing sensitivity, robustness or precision. The design is optimized for in-plate, radioisotopic detection via microplate scintillation counting, featuring compatibility with coincidence counting technology, thereby making it possible to achieve the same high sensitivity as attained with a 96-well plate. Furthermore, the receptor-ligand binding assays are performed directly in the filter plate decreasing the number of manipulations and reducing radioactive solid waste by eliminating the need for a separate incubation plate.
Using parallel experimental procedures, reagents and receptor systems, data are presented that demonstrate the scalability of radiometric receptor-ligand binding assays from a 96- to a 384-well format. The higher density of the 384-well filter plate allows for quantitative receptor-ligand binding assays in a robust, fast and reagent saving format.
Methods and Materials
MultiScreen (Millipore, FB glass fiber, cat. MAFB N0B) and MultiScreenHTS 384 (Millipore, cat. MZFB N0W) filter plates were pretreated with 0.1% polyethyleneimine (PEI) and washed with appropriate assay buffer.
Saturation Binding
8.75 µg (96-well) or 4.38 µg (384-well) of a Human muscarinic M1 receptor expressing transgenic CHO cell membrane fragment bound receptor preparation (PerkinElmer, cat.
RBHM1M) was incubated [200 µL (96-well), 100 µL (384-well)] with serial dilutions of radiolabeled ligand (3H-scopolamine (PerkinElmer, NEN, cat. NET636). After 1 hour incubation at room temperature in the filter plate, the plates were filtered at 12” Hg and washed 10 times by successive filling with binding buffer and filter. The membranes were dried completely before the addition of Opti-Fluor Scintillation cocktail (PerkinElmer, cat. 6013199). Scintillation counting and analysis were performed on a Wallac Microbeta® TriLux in default coincidence counting mode. Nonspecific binding was determined in a separate experiment with an excess (10 µM) of unlabeled competitor ligand [pirenzipine (Sigma, cat. P7412)]. Specific binding was calculated as non-specific activity subtracted from total activity. Binding constants (Kd) were determined by fitting specific binding by free ligand concentration by non-linear regression and Scatchard analysis (not shown) using Prism data software (www.graphpad.com).
Displacement Binding
Receptor amounts used were the same as for above. Radioligand binding inhibition was determined with a constant radioligand concentration (0.6 nM 3H-scopolamine) and serial dilutions of unlabeled competitor ligand (pirenzipine) as compared to a control binding experiment without unlabeled ligand (% Control). Relative affinity values (IC50) were determined by fitting binding inhibition values by non-linear regression using Prism data software.

Figure 1.Saturation binding results of scopolamine binding experiments to the Human muscarinic M1 on polyethyleneimine (PEI) treated MultiScreenHTS 384-well FB filter plates. Non-specific binding was determined in a separate experiment in an excess of the unlabeled muscarininc receptor antagonist, pirenzipine (10 µM). Specific binding was calculated as non-specific binding subtracted from total activity. All data points are the average of triplicate assays.
Results
Binding Affinity Determination of Scopolamine to the Human Muscarinic M1 GPCR by Saturation Binding: Correlation of MultiScreen 96-well and MultiScreenHTS 384-well FB Filter Plates
Saturation receptor-ligand binding was performed with the Human muscarinic M1 receptor and serial dilutions of tritium labeled scopolamine (Figure 1). In a separate parallel assay, an excess of an unlabeled ligand is used to determine non-specific binding (NSB). Saturation binding assays are ideal for the determination of the specific activity of the receptor preparation (Bmax) and the binding affinity constant (Kd) for the binding of the ligand to the receptor.
The measured binding affinity for scopolamine was Log Kd = –9.3 (0.5 nM) using 384-well MultiScreenHTS filter plates (Table 1), in good agreement with literature values3,4. The specific receptor activity of the cell membrane receptor preparation was determined by the fitted Bmax value of 3285 ± 164 fmol/mg of total protein. This value is in the order of the typical specific activity range of 1.5 to 3 pm/mg listed by the supplier (PerkinElmer).
For direct comparison, parallel binding assays with the Human muscarinic M1 receptor were performed in the MultiScreen 96-well FB filter plate (Figure 2). Absolute counts (CPM) of the binding assays were observed to be higher in the 96-well format than for the 384-well format. Normalization for this difference was accomplished by using the coincidence counting efficiencies of the two plates (7.9% for 96-well, 2.6% for 384-well). Binding parameters determined in the 96-well FB filter plate were Log Kd = –9.1 and Bmax = 3288 ± 129 fmol/mg total protein (Table 1). Both values are in excellent correlation with those determined in the MultiScreenHTS 384-well FB filter plate.
Antagonist Displacement Binding of the GPCR Human Muscarinic M1 Receptor using MultiScreenHTS 384-well FB Filter Plates
Displacement binding is a method in which a radiolabeled ligand specifically bound to a receptor is displaced by a non-labeled competitor ligand. This is accomplished by a titration of the non-labeled ligand to either estimate the relative binding affinity for the receptor (IC50 or Ki, the concentration of non-labeled competitor which displaces 50% of the radioligand) or to screen large libraries of compounds for their ability to displace a bound radioligand.
Displacement of scopolamine binding was performed in the MultiScreenHTS 384-well FB filter plate in a 100 µL assay volume using non-labeled pirenzipine, an antagonist for the muscarinic M1 receptor2 (Figure 3, top). For a direct comparison, displacement binding was also performed in the MultiScreen 96-well FB filter plate in a 200 µL assay volume (Figure 3, bottom). The Log IC50 values of –6.9 and –7.0 (384-well and 96-well respectively) for displacement of scopolamine binding of the muscarinic M1 receptor by pirenzipine was determined.
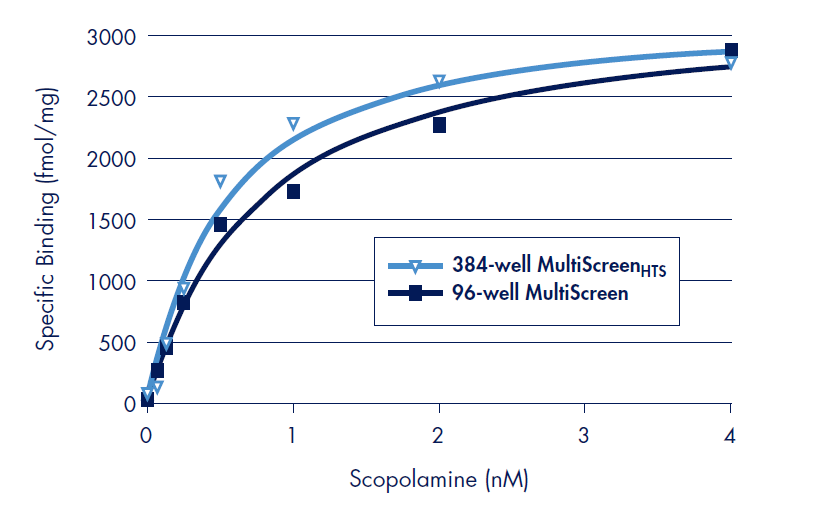
Figure 2.Results of scopolamine binding experiments to the Human muscarinic M1 on polyethyleneimine (PEI) treated Barex MultiScreen 96-well and MultiScreenHTS 384-well FB filter plates. 8.75 µg (96-well) or 4.38 µg (384-well) receptor preparation was incubated with serial dilutions of radiolabeled scopolamine. Binding constants (Kd) were determined by fitting specific binding by free scopolamine concentration by non-linear regression and Scatchard analysis (not shown) using Prism data software (www. graphpad.com). All data point are the average of triplicate experiments.
Discussion
In this study, in-plate receptor-ligand binding assays were performed in both MultiScreen 96-well and MultiScreenHTS 384-well FB filter plate formats with a G-protein Coupled Receptor (GPCR), the Human muscarinic M1 receptor. Accurate and reproducible determinations of receptor specific activity (Bmax), binding affinity (Kd) and IC50 values of competitor ligands were measured. Values performed on 96-well and 384-well FB glass fiber plates were shown to be in excellent agreement (Table 1).
For binding experiments in the 96-well filter plate, 8.75 µg of total protein was used in a 200 µL binding reaction. This volume is typical for 96-well binding assay to minimize the receptor concentration preventing ligand depletion while retaining enough receptor mass to achieve a good signal. In contrast, the 384-well binding assays were performed in half the volume (100 µL) with 4.38 µg total protein.
The design of the MultiScreenHTS 384-well filter plate allows for accurate coincidence counting at very low levels of radioactivity permitting the use of less receptor mass, thus preventing ligand depletion in smaller assay volumes (Figure 4). Thus, cost savings through the use of less reagents can be achieved without sacrificing sensitivity
Displacement binding is the typical mode of screening large libraries of compound for their ability to displace a known ligand. Robust displacement binding in the MultiScreenHTS 384 has been shown to be equivalent to the standard 96-well filter plate format (Figure 3). With the higher density format of the MultiScreenHTS 384-well filter plate, higher throughput of library screening can be achieved.
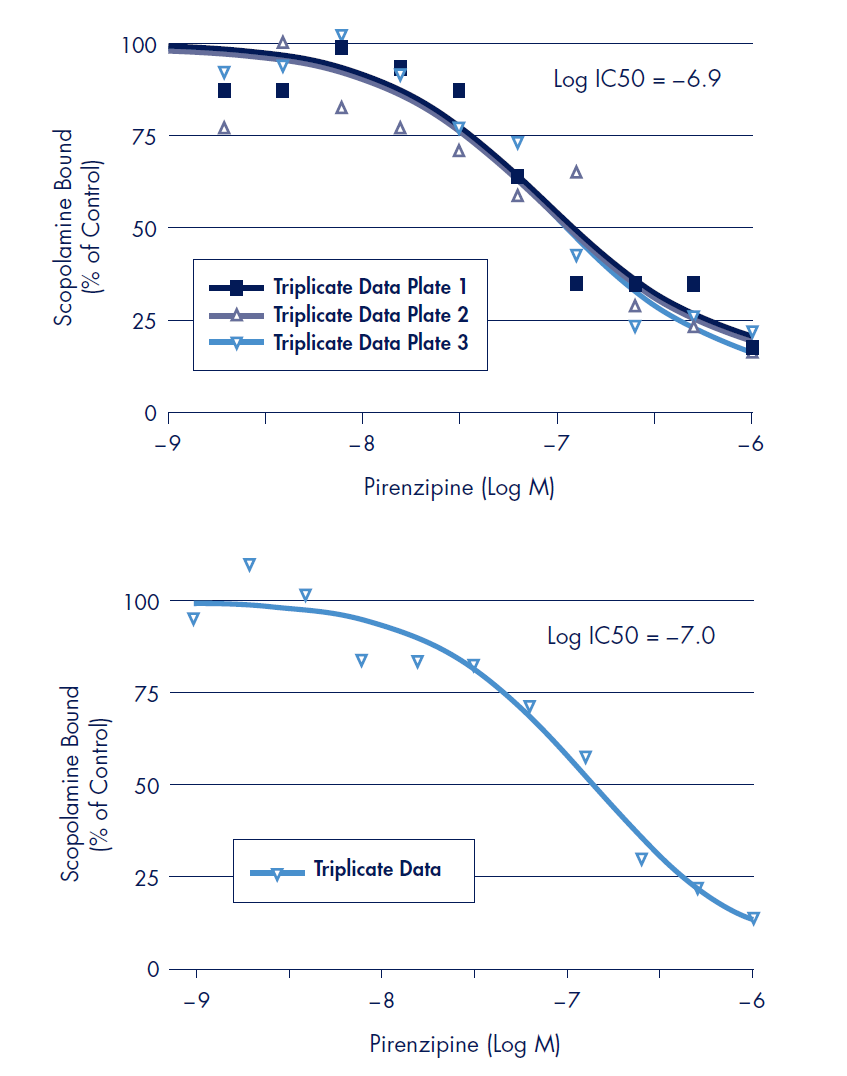
Figure 3.Radioligand displacement binding assays were performed with a constant radiolabeled scopolamine concentration (0.6 nM) and serial dilutions of unlabeled pirenzipine as compared to a control binding experiment without unlabeled pirenzipine (% control). Top: Displacement binding done with 4.38 µg receptor preparation in 100 µL using MultiScreenHTS 384-well filter plate. Results presented are from three separate experiments each performed in triplicate wells. Bottom: Displacement binding done with 8.75 µg receptor preparation in 200 µL using MultiScreen 96-well filter plate. Relative affinity values (IC50) were determined by fitting displacement binding inhibition values by non-linear regression using Prism data software. All data points are the average of triplicate experiments.
Conclusions
High throughput screening of receptor-ligand binding can be achieved using the MultiScreenHTS 384-well filter plate. The ability to reproducibly measure precise quantitative binding data, by optimizing the 384-well filter plate for coincidence counting of very low levels of radioactivity, as well as perform screening type binding assays in the 384-well format is shown to be equivalent to the MultiScreen 96-well filter plate format.
Receptor ligand binding in the 384-well filter plate can be performed with half the reagents in half the assay volume as the 96-well format with the same robustness and reproducibility saving assay cost through the use of less reagent. In addition, receptor-ligand binding assays can be performed directly in the MultiScreenHTS 384-well filter plate decreasing the number of manipulations, and reducing radioactive solid waste by eliminating the need for a separate incubation plate.
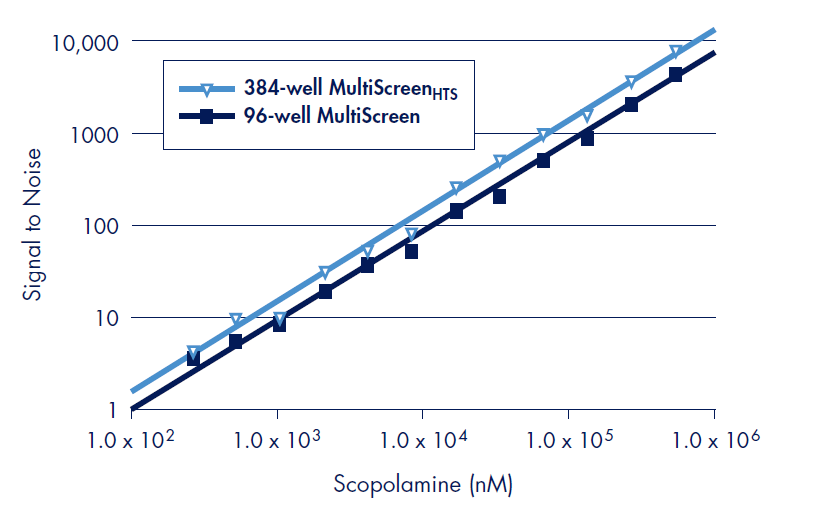
Figure 4.The design of the MultiScreenHTS 384-well filter plate allows for radioacticity detection, linear over several orders of magnitude of signal, equivalent to the 96-well MultiScreen filter plate. Signal to noise was determined using the 3H-labeled ligand WIN 35,428 by loading indicated disintegrations per minute (DPM) of reagent to duplicate wells for the MultiScreenHTS 384- and the MultiScreen 96-well filter plates with FB glass fiber in the Wallac MicroBeta TriLux liquid scintillation counter in default coincidence counting mode. The measured CPM values were divided by the average CPM of the remaining wells that did not contain radioactive material in order to determine signal to noise.
References
Materials
To continue reading please sign in or create an account.
Don't Have An Account?