Purification or Removal of Proteins and Peptides with Exposed Amino Acids: His, Cys, Trp, and/or with Affinity for Metal Ions
Chelating Sepharose High Performance, Chelating Sepharose Fast Flow, Capto Chelating
Proteins and peptides that have an affinity for metal ions can be separated using immobilized metal-ion affinity chromatography, IMAC. The metals are immobilized onto a chromatographic medium by chelation. Certain amino acids, for example, histidine and cysteine, form complexes with the chelated metals around neutral pH (pH 6.0 to 8.0) and it is primarily the histidine-content of a protein which is responsible for it’s binding to a chelated metal.
IMAC is excellent for purifying recombinant (his)6-tagged proteins (see the handbook Affinity Chromatography Vol. 2: Tagged Proteins, 18114275) as well as many natural proteins. Chelating Sepharose, the medium used for IMAC purification of proteins with exposed His, Cys, and Trp, is formed by coupling a metal chelate forming ligand (iminodiacetic acid) to Sepharose.
Before use the medium is loaded with a solution of divalent metal ions such as Ni2+, Zn2+, Cu2+, or Co2+. The binding reaction with the target protein is pH dependent and bound sample is eluted by reducing the pH and increasing the ionic strength of the buffer or by including imidazole in the buffer. The structure of the ligand, iminodiacetic acid, is shown in Figure 1.
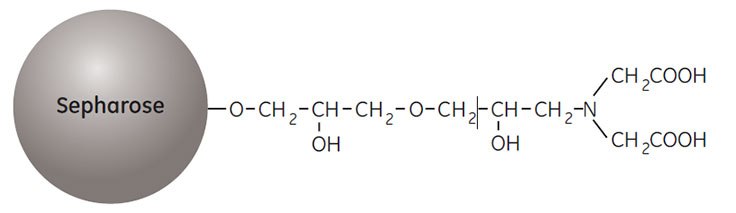
Figure 1.Partial structure of Chelating Sepharose High Performance and Chelating Sepharose Fast Flow.
Metalloproteins are not usually suitable candidates for purification by chelating chromatography since they tend to scavenge the metal ions from the column.
Chromatography Media Characteristics
1 Short term refers to the pH interval for regeneration, cleaning-in-place, and sanitization procedures. Long term refers to the pH interval over which the medium is stable over a long period of time without adverse effects on its subsequent chromatographic performance.
Purification Options
1 See Appendix 4 to convert flow velocity (cm/h) to volumetric flow rate (ml/min). Maximum operating flow is calculated from measurement in a packed column with a bed height of 10 cm and i.d. of 5 cm.
Selecting the Metal Ion
The following guidelines may be used for preliminary experiments to select the metal ion that is most useful for a given separation:
- Cu2+ gives strong binding and some proteins will only bind to Cu2+. Load metal-ion solution equivalent to 60% of the packed column volume during charging to avoid leakage of metal ions during sample application. Alternatively, the medium can be saturated and a short secondary uncharged column of HiTrap® Chelating HP or packed Chelating Sepharose Fast Flow should be connected in series after the main column to collect excess metal ions.
- Zn2+ gives a weaker binding and this can, in many cases, be exploited to achieve selective elution of a protein mixture. Load metal-ion solution equivalent to 85% of the packed column volume to charge the column.
- Ni2+ is commonly used for his-tagged proteins. Ni2+ solution equivalent to half the column volume is usually sufficient to charge the column.
- Co2+ and Ca2+ are also alternatives.
Charge the column with metal ions by passing through a solution of the appropriate salt through the column, for example, ZnCl2, NiSO4, or CuSO4 in distilled water. Chloride salts can be used for other metals.
Several methods can be used to determine when the column is charged. If a solution of metal salt in distilled water is used during charging, the eluate initially has a low pH and returns to neutral pH as the medium becomes saturated with metal ions. The progress of charging with Cu2+ is easily followed by eye (the column contents become blue). When charging a column with zinc ions, sodium carbonate can be used to detect the presence of zinc in the eluate. Wash the medium thoroughly with binding buffer after charging the column.
Choice of Binding Buffer
A neutral or slightly alkaline pH will favor binding. Tris-acetate (50 mM), sodium phosphate (20 to 50 mM) and Tris-HCl (20 to 50 mM) are suitable buffers. Tris-HCl tends to reduce binding and should only be used when metal-protein affinity is fairly high.
High concentrations of salt or detergents in the buffer normally have no effect on the adsorption of protein and it is good practice to maintain a high ionic strength (e.g., 500 mM to 1 M NaCl) to avoid unwanted ion exchange effects.
Chelating agents such as EDTA or citrate should not be included as they will strip the metal ions from the medium.
Choice of Elution Buffers
Differential elution of bound substances may be obtained using a gradient of an agent that competes for either the ligand or the target molecules. An increased concentration of imidazole (0 to 500 mM), ammonium chloride (0 to 150 mM), or substances such as histamine or glycine with affinity for the chelated metal can be used. The gradient is best run in the binding buffer at constant pH.
Since pH governs the degree of ionization of charged groups at the binding sites, a gradient or stepwise reduction in pH can be used for nonspecific elution of bound material. A range of pH 7.0 to 4.0 is normal, most proteins eluting between pH 6.0 and 4.2. Deforming eluents such as 8 M urea or 6 M guanidine hydrochloride can be used.
Elution with EDTA (50 mM) or other strong chelating agents will strip away metal ions and other material bound. This method does not usually resolve different proteins.
If harsh elution conditions are used, it is recommended to transfer eluted fractions immediately to milder conditions (either by collecting them in neutralization buffer or by passing directly onto a desalting column for buffer exchange (see Buffer exchange and desalting, Appendix 1).
The loss of metal ions is more pronounced at lower pH. The column does not have to be stripped between consecutive purifications if the same protein is going to be purified.
Although metal-ion leakage is very low, the presence of any free metal in the purified product can be avoided by connecting an uncharged HiTrap® Chelating HP column in series after the first column and before the protein is eluted. This column will bind any metal ions removing them from the protein as it passes through the second column.
Performing a Separation
This protocol can be used as a base from which to develop purification methods for proteins and peptides with affinity for metal ions:
- Metal-ion solution: 100 mM CuSO4
- Binding buffer: 20 mM sodium phosphate, 500 mM NaCl, 10 mM imidazole, pH 7.4
- Elution buffer: 20 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, pH 7.4
Use water, not buffer, to wash away the column storage solution which contains 20% ethanol. This avoids the risk of nickel salt precipitation in the next step. If air is trapped in the column, wash the column with distilled water until the air bubbles are expelled.
- Wash the column with at least 2 CV of distilled water.
- Load 0.5 CV of the 100 mM copper solution onto the column.
- Wash with 5 CV of distilled water.
- Equilibrate the column with 10 CV of binding buffer.
- Apply sample at a flow rate of 1 to 4 ml/min (1 ml column) or 5 ml/min (5 ml column). Collect the flowthrough fraction. A pump is more suitable for application of sample volumes greater than 15 ml.
- Wash with 10 CV of binding buffer. Collect wash fraction.
- Elute with 5 CV of elution buffer. Collect eluted fractions in small fractions such as 1 ml to avoid dilution of the eluate.
- Wash with 10 CV of binding buffer. The column is now ready for a new purification and there is rarely a need to reload with metal if the same (his)6-tagged protein is to be purified.
Scaling Up
To increase capacity, use several HiTrap® Chelating HP columns (1 ml or 5 ml) in series (note that back pressure will increase) or, for even larger capacity, pack Chelating Sepharose Fast Flow into a suitable column (see Appendix 3).
Cleaning
- Remove metal ions by washing with 5 CV of 20 mM sodium phosphate, 500 mM NaCl, 50 mM EDTA, pH 7.4
- Remove precipitated proteins by filling the column with 1 M NaOH and incubate for 2 h. Wash out dissolved proteins with 5 CV of water and a buffer at pH 7.0 until the pH of the flowthrough reaches pH 7.0.
- Alternatively wash with a nonionic detergent such as 0.1% Tween 20 at 37 °C for 1 min.
- Remove lipid and very hydrophobic proteins by washing with 70% ethanol, or with a gradient 0%–30%–0% isopropanol/water.
Chemical Stability
Stable in all commonly used aqueous buffers and denaturants such as 6 M guanidine hydrochloride, 8 M urea, and other chaotropic agents.
Storage
Wash chromatography media and columns with 20% ethanol at neutral pH (use approximately 5 CV for packed media) and store at 4 °C to 8 °C.
Before long-term storage, remove metal ions by washing with 5 CV of 20 mM sodium phosphate, 500 mM NaCl, 50 mM EDTA, pH 7.4.
The column must be recharged with metal ions after long-term storage.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?