Glycoprotein Deglycosylation
Aparagine-linked (N-linked) and serine/threonine-linked (O-linked) oligosaccharides are major structural components of many eukaryotic proteins. They perform critical biological functions in protein sorting, immune recognition, receptor binding, inflammation, pathogenicity, and many other processes. The diversity of oligosaccharide structures, both O-linked and N-linked, often results in heterogeneity in the mass and charge of glycoproteins. Variations in the structure and different degrees of glycosylation site saturation in a glycoprotein contribute to mass heterogeneity. The presence of sialic acid (N-acetyl neuraminic acid) also affects both the mass and charge of a glycoprotein. N-linked oligosaccharides may contribute 3.5 kDa or more per structure to the mass of a glycoprotein (Figure 1). O-linked sugars, although usually less massive than N-linked structures, may be more numerous (Figures 2 and 3).
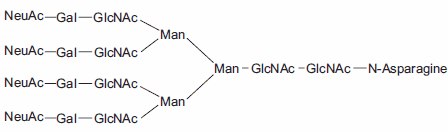
Figure 1.Tetraantennary N-linked Sugar.

Figure 2.Di- and Trisialyated O-linked Core-1 Saccharides (core shown in bold).

Figure 3.O-linked Core-2 Hexasaccharide.
Abbreviations:
Gal – Galactose, Man – Mannose, GalNAc – N-acetylgalactosamine, GlcNAc – N-acetylglucosamine, NeuAc – N-acetylneuraminic acid (Sialic acid)
To study the structure and function of a glycoprotein, it is often desirable to remove all or a select class of oligosaccharides. This allows assigning specific biological functions to particular components of the glycoprotein. For example, the loss of ligand binding to a glycoprotein after removal of sialic acid may implicate that sugar in the binding process.
Removing carbohydrate groups from glycoproteins is highly recommended for protein identification. Glycoproteins and glycopeptides ionize poorly during mass spectrometry (MS) analysis, leading to inadequate spectral data. Glycopeptides have lower detection sensitivity due to microheterogeneity of the attached glycans, resulting in signal suppression. Proteolytic (tryptic) digestion of native glycoproteins is often incomplete due to steric hindrance from the presence of bulky oligosaccharides. However, proteolytic cleavage is a prerequisite when eluting peptide fragments from gels for identification by MS. Deglycosylation of the glycopeptides before tryptic digestion increases protein sequence coverage and improves protein identification, as well as aids in identifying glycosylation sites on the protein core.
Kits for Chemical and Enzymatic Deglycosylation of Glycopeptides
- Chemical deglycosylation using trifluoromethanesulfonic acid (TFMS) hydrolysis leaves an intact protein component, but destroys the glycans. Glycoproteins from animals, plants, fungi, and bacteria have been deglycosylated by this procedure. It has been reported that the biological, immunological, and receptor binding properties of some glycoproteins are retained after deglycosylation by this procedure, although this may not be true for all glycoproteins. The reaction is non-specific, removing all types of glycans regardless of structure, although prolonged incubation is required for complete removal of O-linked glycans. Also, the innermost Asn-linked GlcNAc residue of N-linked glycans remains attached to the protein. This method removes the N-glycans of plant glycoproteins that are usually resistant to enzymatic hydrolysis.
- Enzymatic deglycosylation is recommended for use with N-linked glycans and can be combined with tryptic digestion. Use of the glycolytic enzyme PNGase F is the most effective method of removing virtually all N-linked oligosaccharides from glycoproteins. Peptide-N-glycosidase F (PNGase F) releases asparagine-linked oligosaccharides from glycoproteins and glycopeptides by hydrolyzing the amide group of the asparagine (Asn) side chain. A tripeptide with the oligosaccharide-linked asparagine as the central residue is the minimum substrate for PNGase F. The oligosaccharides can be high mannose, hybrid, or complex type. However, N-glycans with fucose linked α(1→3) to the asparagine-bound N-acetyl glucosamine are resistant to the action of PNGase F (Figure 4).
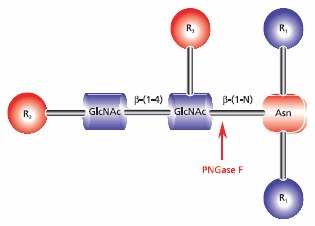
Figure 4.Cleavage site requirements for PNGase F. R1 = N- and C- substitution by groups other than H, R2 = H or the rest of an oligosaccharide , R3 = H or α(1→6) fucose.
GlycoProfile™ IV Chemical Deglycosylation Kit
The optimized GlycoProfile IV Chemical Deglycosylation Kit removes glycans from glycoproteins using trifluoromethanesulfonic acid (TFMS). The deglycosylated protein can then be recovered using a suitable downstream processing method such as gel filtration or dialysis. Unlike other chemical de glycosylation methods, hydrolysis with anhydrous TFMS is very effective at removing O- and N-linked glycans (except the innermost Asn-linked GlcNAc of N-linked glycans) with minimal protein degradation. The extent of deglycosylation may be assessed by mobility shift of the deglycosylated protein versus the intact glycoprotein on SDS-PAGE gels (Figure 5).
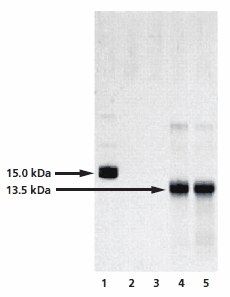
Figure 5.Analysis of the chemical deglycosylation of RNase B on 12% homogeneous SDS-PAGE gel. Lane 1 is the RNase B control (Product No. R1153), while lanes 2 to 5 represent fractions collected from the gel filteration column. Lanes 2 and 3 are pre-void volume fractions and lanes 4 and 5 show bands at 13.5 kDa, corresponding to deglycosylated RNAse B.
- Each reaction processes 1-2 mg of glycoprotein – Enough output for downstream analysis
- Minimal degradation of protein core – For more reliable MS data
- Complete deglycosylation in as short as 30 minutes – For increased throughput
Enzymatic Protein Deglycosylation Kit
The Enzymatic Protein Deglycosylation Kit contains all the enzymes and reagents needed to completely remove all N-linked and simple O-linked carbohydrates from glycoproteins, and effect cleavage of complex core-2 O-linked carbohydrates, including those containing polylactosamine.
PNGase F (Peptide-N-glycosidase F) is included for N-linked deglycosylation of glycoproteins and glycopeptides in solution, in-gel digests, or on blot membranes. The enzyme releases asparagine-linked oligosaccharides from glycoproteins and glycopeptides by hydrolyzing the amide group of the asparagine (Asn) side chain.
For degradation of O-linked glycans, monosaccharides must be removed by a series of exoglycosidases until only the Gal- β(1→3)-GalNAc core remains attached to the serine/threonine. The EDEGLY kit contains α(2→3,6,8,9)-Neuraminidase (Sialidase A) for cleavage of terminal sialic acid residues, and O-Glycosidase to remove the core Gal-β(1→3)-GalNAc. β(1→4)-Galactosidase and β-N-Acetyl glucos aminidase are also provided to remove sugars associated with specific O-linked glycan structures.
- Deglycosylates up to two mg of glycoprotein – Sufficient for downstream processing
- Single reaction at neutral pH – Retain original peptide structure
- No protein degradation – Perform interrogation on peptide structure
- Removes O-linked sugars containing polysialic acid – Get more accurate peptide analysis
- Control glycoprotein provided – Verification improves confidence and consistency
GlycoProfile™ I Enzymatic In-Gel N-Deglycosylation Kit
The GlycoProfile I Enzymatic In-Gel N-Deglycosylation Kit robustly removes N-linked glycans and digests protein samples from 1D- or 2D-polyacrylamide gels for MS or HPLC analysis. The kit works well for Coomassie Brilliant Blue, colloidal Coomassie and silver stained gels when properly destained. The glycolytic enzyme PNGase F (Peptide-N-glycosidase F) performs superbly when used for in-gel N-linked de glycosy lation of glycoproteins and glycopeptides. Proteomics Grade Trypsin effectively digests the remaining protein. Desalted samples are then concentrated for analysis by MALDI-TOF-MS or ES-MS (Figure 6).
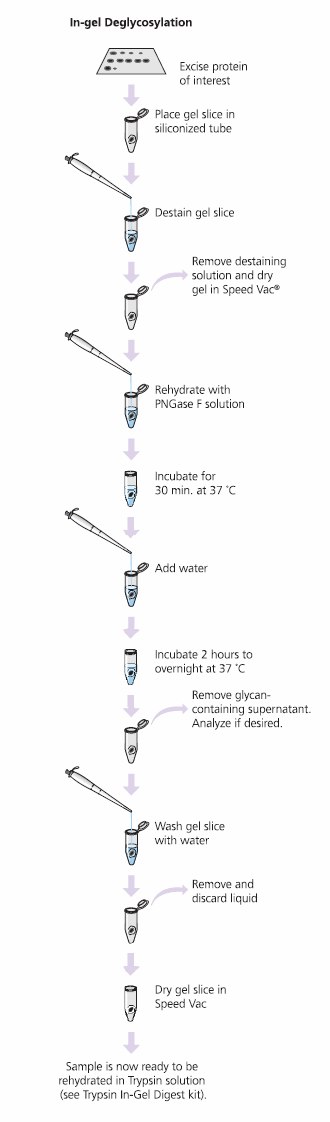
Figure 6.Process for the GlycoProfile I Enzymatic In-Gel N-Deglycosylation Kit. Glycoprotein Deglycosylation
- In-gel deglycosylation and digestion – Minimizes sample manipulation
- Highly purified enzymes – Prevents unwanted activities and byproducts
- Low buffer salt content – Eliminates interference with MS analysis
- Destaining reagent included – Saves time by reducing additional reagent preparation
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?