Egg-based and Cell-based Influenza Vaccine Manufacturing Methods
Influenza virus is an enveloped single-stranded RNA virus that causes influenza (flu). There are three serotypes of seasonal influenza virus—A, B, and C. While type A affects humans and non-humans, type B affects only humans, and type C occurs much less frequently and affects humans very rarely. Type A viruses are the most virulent pathogens among the three types; they cause the most severe disease in humans.
Antigens Targeted in Influenza Vaccines
The differences in these types of influenza viruses are based on the antigenic differences of the two internal structural proteins, nucleocapsid (NP) and matrix (M) proteins. These proteins have no cross reactivity among the three types. Subtyping of the virus is done by the antigenic variations in the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA). About fourteen subtypes of HA (H1–H14) and nine subtypes of NA (N1–N9) have been recovered so far, in different combinations, from birds and mammals, including humans. Three HA subtypes (H1–H3) and two NA subtypes (N1, N2) have been recovered from humans.
Hemagglutinin (HA, MW ~77 kDa) is an immunogenic protein located at the surface of the virus envelope. Neuraminidase (NA, MW ~220 kDa) is a surface enzyme protein. These proteins are the antigens that define the particular strain of influenza and play critical roles in mediating the entry of the virus into the target cell. The HA protein is involved in attachment and membrane fusion in the endosome of the infected cell. The antigenic domains are on the surface and can be altered. The virus can thus prevent the triggering of an immune response and still maintain the ability to bind to the receptor.
Influenza virus undergoes frequent minor genetic mutations known as “antigenic drift.” This is defined as subtle changes in the antigenic proteins on the virus surface that allow the viruses to evade host immunity and cause disease despite previous infection or immunization. Large antigenic shift changes in the type A virus antigens happen about every ten years, resulting in larger epidemics, or pandemics. Seasonal influenza vaccines are usually trivalent, and more recently tetravalent, pandemic vaccines are monovalent.
Vaccine manufacturers typically require months for the development and production of a seasonal influenza vaccine each year. The production of the influenza vaccine can be done using various methods including egg-based vaccine manufacturing and cell-based vaccine manufacturing.
Cell-based Influenza Vaccines vs. Egg-based Influenza Vaccine Production
Most influenza vaccines are egg-based. However, there is a long lead time to secure chicken eggs every year. Use of cell culture–based influenza vaccine manufacturing eliminates this bottleneck and the possibility of contamination with the avian flu virus, which can originate from eggs.
The cell culture–based manufacturing process is also more reproducible as different influenza strains grow at different rates in eggs, which leads to variability in yield. Cell culture–derived viruses are also of higher initial purity, and the absence of egg-based proteins (collagens and albumins) presents advantages in purification of the inactivated harvest.
1. Cell-based Influenza Vaccine Manufacturing Process
There is no template process for cell culture–based influenza vaccine, and manufacturers follow different methods of manufacturing and select various technologies for their own process philosophies. A typical cell culture–based flu vaccine process is shown in Figure 1.
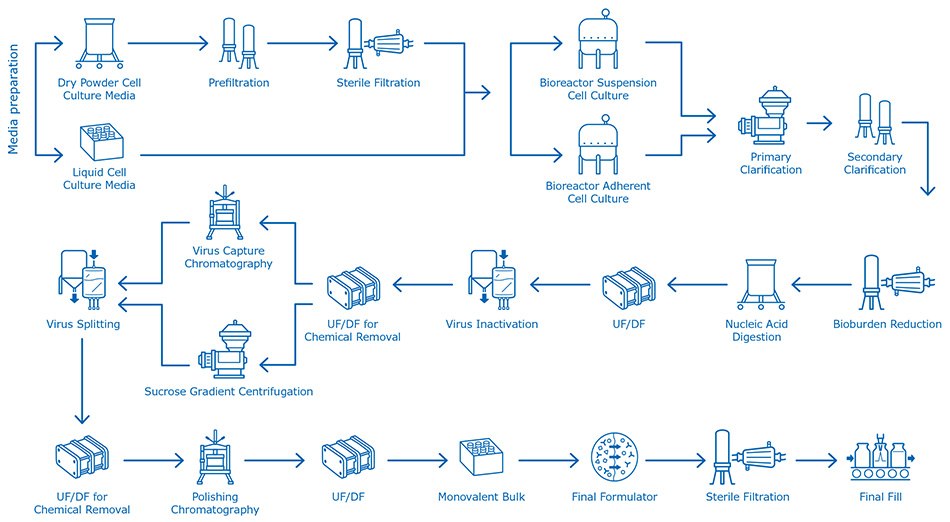
Figure 1.Cell culture-based influenza vaccine manufacturing process, from media preparation, cell culture growth, then clarification, nucleic acid removal, virus inactivation and all further downstream purification steps, up to final fill.
Typical commercial bioreactor sizes range from 2,500–5,000 L. Typical total process yield is approximately 35% and is highly dependent on the virus strain. Based on this yield and recovery, a total of 50–100 doses of vaccine can be produced per liter of cell culture.
Cell-based Propagation and Growth of Influenza Virus
Successful infection requires the addition of proteases to the medium, preferably trypsin or a similar serine proteases. These proteases extracellularly cleave the precursor protein of hemagglutinin (HA0) into active hemagglutinin (HA1 and HA2). Only cleaved hemagglutinin leads to the adsorption of the influenza viruses on cells with subsequent virus assimilation into the cell, which leads to further replication. Passaging of the MDCK cells requires trypsinization with trypsin enzyme, which is stopped using an equimolar volume of trypsin inhibitor solution. Cells from roller bottles are used to inoculate 3-liter bioreactors and single-use bioreactors (SUBs).
MDCK cell culture can produce up to 1x109 pfu/mL of influenza virus upon infection and incubation for three to five days. Parameters such as multiplicity of infection (MOI), incubation time, and temperature must be optimized for each cell line and each strain of virus. Assuming 45 µg of HA per dose (0.5 mL), it is likely that a 1,000-L to 2,000-L bioreactor (with microcarrier-based MDCK suspension culture) would produce 20 million doses per season. It is estimated that production from an optimized 1,000-L bioreactor using solid microcarriers and MDCK cells would be comparable to production from approximately 31,000 eggs.
Clarification
During the clarification, cell or cell debris are removed. Depending on which technology is used for the clarification, smaller impurities such as HCP’s and DNA may also be partially removed. The solid content in viral vaccine harvest is generally low, a single-stage (primary) clarification is therefore usually sufficient.
Commonly used technologies for the (primary) clarification of viral vaccines include: centrifugation, microfiltration tangential-flow filtration (MF-TFF), and depth filtration.
Centrifugation can be a more economical solution for large-scale production (≥2,000L bioreactor scales). However, centrifuge scale-up and operation can be a hassle, and the capital expenditure to acquire a centrifuge is high. Some manufactures use MF-TFF (with 0.45 µm or 0.65 µm non-screened membranes) at low shear conditions for the clarification. The implementation and operation of MF-TFF is quite cumbersome for process development and for operators at manufacturing scale. In addition, yield is typically low ~50%. Depth filtration is commonly the preferred method of choice. When using depth filters, it is important to be aware that these filters are often positively charged. As influenza virus and the host cell DNA are negatively charged, positive-charged filters sometimes produce high adsorption of virus along with good DNA removal. It is essential to optimize this adsorption tradeoff using filtration flux and by carefully selecting buffer conditions (salt, pH, etc.). With proper optimization, recoveries of >90% can be achieved.
At the secondary clarification step, a 0.22 µm sterile filtration is usually preferred over a 0.45 µm bioburden reduction filtration.
Virus Inactivation
Virus inactivation can be done using formaldehyde or UV radiation.
Formaldehyde is the most frequently used inactivating agent in vaccine manufacturing. Formaldehyde inactivates a virus by irreversibly crosslinking primary amine groups in surface proteins with nearby nitrogen groups in DNA or proteins. These crosslinking bonds can associate with non-viral proteins; as a result, initial partial purification of live infectious virus is required to prevent irreversible chemical bridging between viral proteins and impurities. Inactivation is carried out at 32 °C for 24 hours with formalin concentrations of 0.1%.
UV radiation at a wavelength of 254 nm can also be used to inactivate the virus. The effective dose depends on various factors such as size and diameter of the UV lamp, distance between the UV source and virus-containing medium, UV light intensity, and the exposure time for the virus-containing medium. The general dose is 5-200 mJ/cm2.
Ultrafiltration/Diafiltration (UF/DF)
At this stage, whole virus particles are concentrated in order to reduce the process volume downstream. UF/DF is used to remove low-molecular-weight impurities and for buffer exchange. There are multiple UF/DF steps in a cell culture flu vaccine process. UF/DF is implemented to remove DNA, host cell protein (HCP), and Benzonase® endonuclease, and at the post-ultracentrifugation step, to remove sucrose. A 300 kDa device works well for volumetric concentration.
Typical UF/DF conditions are:
- 10–15 psi transmembrane pressure (TMP)
- 5–6 L/min/m2 feed flow
- 20–50X concentration factor
- 50 LMH average permeate flux
- Due to a high concentration factor, some processes are performed in a fed batch concentration mode.
500 kDa with flux control operation is used to remove host cell DNA and proteins. A TFF process results in good low-molecular-weight DNA removal but is less efficient to remove high-MW molecules. In some cases, a 1000 kDa filter results in good virus retention, depending on the size of the virus strain. In permeate controlled two pump-based TFF, typical average filtration flux is 20–35 LMH at feed flow rate of 5–6 L/in/m2 and TMP of 4–5 psi.
Density Gradient Centrifugation
Either density gradient (zonal) centrifugation or chromatography is commonly used to further purify the virus. Zonal centrifugation is done in two steps: pelleting and fractionation. While zonal centrifugation produces a product with high purity, the yield is low and operation is cumbersome.
Chromatography
In the cell culture–based process, size exclusion and anion exchange chromatography are performed on inactivated virus to remove DNA and HCP. Due to the large size of the virus, the anion exchange chromatography step is operated in flow-through mode. In some cases, size exclusion chromatography (SEC) is followed by anion exchange chromatography (AEX). SEC is generally used to remove small solutes and proteins, but separation from nucleic acid is difficult to achieve. AEX resin is used with NaCl at sufficient concentration such that the influenza virus does not bind to the resin while nucleic acid and other impurities do bind to the resin. Although the size of hcDNA and the influenza virions is similar, the random coil of DNA promotes stronger binding to the resin compared with the binding of rigid sphere of virions.
Adding detergent to prevent aggregation of virions can improve purity (lower hcDNA) and product yield. The average product yield in the SEC step is 85% with 30– 35% reduction in total protein content and nucleic acid. An average product yield from the anion exchange step is more than 80% with an approximately 60–70-fold reduction of nucleic acid. The overall product yield from chromatographic purification is 50–55% with a 15–20- fold reduction in total protein and more than a 500-fold reduction in nucleic acid.
DNA Removal with Nuclease Treatment
The U.S. Food and Drug Administration (FDA) requires that a parenterally administered dose is limited to 100 pg of residual host DNA. The European Medicines Agency (EMA) and World Health Organization (WHO) allow 10 ng per parenteral dose and 100 μg per dose for orally administered vaccine. Benzonase® endonuclease, a genetically engineered endonuclease, cleaves all forms of DNA and RNA. One unit of Benzonase® endonuclease degrades approximately 37 μg DNA in thirty minutes to as low as 3–8 base pairs (<6 kDa). If not present in the original buffer system, 1–2 mM of MgCl2 is needed for optimal Benzonase® endonuclease performance. DNA presence in feed material depends on the cell/virus types as well as the methods and techniques used at the harvest step. After the Benzonase® endonuclease treatment, a quantitative removal of Benzonase® endonuclease from the process stream is required in the subsequent purification steps. Therefore, it is better to use Benzonase® endonuclease treatment sufficiently upstream. Several methods are used to remove Benzonase® endonuclease from the process: TFF (MW cut-off 300 kDa), anion-exchange chromatography (AEX), and zonal centrifugation. There are reports indicating the use of ~0.9 -1.1 U/mL of Benzonase® endonuclease for treatment of harvest to degrade host cell nucleic acid at 30 to 37 °C in four to eight hours. Due to regulatory requirements, residual Benzonase® endonuclease must be measured and detected in the process. ELISA-based methods are used for this detection.
Splitting Process to Create Split Vaccines
Most influenza vaccines are “split” vaccines, which means they are produced by detergent treatment. This step breaks down the viral envelope and releases the internal antigenic components such as the viral RNA–associated capsid nucleoprotein and the envelope inner protein matrix (M protein). This splitting process removes some of the viral components, which results in a less reactogenic vaccine.
Prior to fragmentation, the concentrated monovalent (single strain) viral suspension is diluted with a sterile buffer.
Viral fragmentation takes place when amphiphilic non-ionic detergent such as Triton® X-100 solution (0.5 %) and/or anionic sodium deoxycholate (DOC) is added to the suspension of the purified influenza. Polysorbates (Tween® 80 polyethylene sorbitol ester) and cetyltrimethyl ammonium bromide (CTAB) are also suitable for the virus splitting step. Fragmentation requires continuous stirring to mix the process fluid with the detergent for at least one hour at room temperature. The length of the fragmentation step can be extended up to twenty-four hours if necessary.
A secondary virus inactivation is employed after the virus splitting process as an additional safety measure to ensure complete inactivation of every component.
Sterile Filtration
When sterile filtration happens in the manufacturing process depends on whether the vaccine is a whole virus vaccine or a split virus vaccine.
Whole virus vaccines: Some cell culture–based influenza vaccines, such as the vaccine for H5N1, are whole virus vaccines. For these viruses, sterile filtration of the final bulk is done at the end of the process.
Split virus vaccines: For split virus vaccines, the final sterile filtration is done after the splitting step. Depending on the influenza strain, the capacity of the final sterile filters is occasionally low (around 20–50 L/m2). In these cases, 0.45 µm or 0.65 µm prefilters are used to protect the sterilizing grade filter and increase its capacity. The capacity of the sterilizing filter is in the range of 200–400 L/m2 depending on the feed quality and prefilter, if one is used. A 0.45/0.22 µm sterilizing filter is also often used as a terminal sterile filter (if prefiltration is deemed necessary) to trim the process from two to one filtration.
Formulation
Only a few split influenza virus vaccines in the cell culture–based influenza vaccine market are formulated with adjuvant. Adjuvants based on oil-in-water emulsions used in commercial influenza vaccine formulation are MF59 and AS03. For example, Optaflu® vaccine, a cell culture–based trivalent seasonal influenza vaccine (split vaccine, inactivated HA and NA), is formulated with adjuvant MF59. A dose of 0.5 mL of Optaflu® vaccine contains total 45 μg HA (15 μg x 3) and 0.25 mL MF59 adjuvant.
Because formulated vaccine cannot be filter sterilized, adjuvant and purified split virus antigens are filter sterilized separately and then aseptically blended. Typically, 0.22 µm filters are used to sterile filter emulsion-based adjuvants. Due to the nature of oil-in-water adjuvants, it is important to use a filter that will prevent breakthrough in necessary bacterial challenge retention testing.
The final formulation often contains a buffering agent (such as sodium citrate dehydrate, citric acid monohydrate, potassium chloride, potassium dihydrogen phosphate, and/or disodium phosphate dehydrate), an isotonic aid (sodium chloride), and a stabilizer (magnesium chloride hexahydrate or calcium chloride dehydrate).
Summary: Cell-based influenza vaccine
Remarkable progress has been made in the production of cell-based influenza vaccine over the past ten to twenty years. Cell-based production provides an innovative method for solving the production bottleneck that often occurs with traditional egg-based influenza production. This makes it not only a valuable alternative method for seasonal influenza vaccine, but also an essential technology as we prepare for the possibility of a pandemic influenza event.
2. Egg-based Influenza Vaccine
Egg-based influenza vaccines represent the majority of influenza vaccines currently offered on the market. With more than thirty years of commercial scale application, it is a well-characterized process. Figure 2 illustrates the generic process to produce inactivated split-virion type of influenza vaccine produced in embryonated chicken eggs.
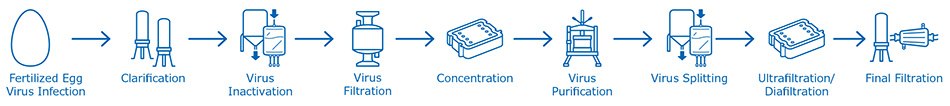
Figure 2.Manufacturing process for producing influenza vaccine in embryonated chicken eggs: from fertilized egg virus Infection, clarification, virus inactivation and all further downstream virus concentration and purification steps, up to final filtration.
Virus Propagation and Harvest
Fertilized chicken eggs are placed into an incubator and kept there at ~37 ºC for nine to twelve days for embryonic development. The allantoises of viable embryos are inoculated with the influenza virus. Antibiotics (typically a mixture of polymyxin and neomycin) are added into the inoculum to suppress potential bacterial growth. The infected eggs are then kept for two to three days at ~33–35 ºC in a humidity controlled incubator to propagate the virus at optimal conditions. The propagation process is stopped by rapid refrigeration of the incubated eggs at ~2–8 ºC. The allantoic fluid rich in viral particles is collected from the infected eggs. To speed up the process and increase capacity, this step of the upstream part of the process is often automated with the use of automatic inoculators, incubators, and harvesters. One egg produces 3 to 10 mL of allantoic fluid. The yield of HA can vary significantly between 0.7 to 3 eggs required per dose of trivalent vaccine, depending on the strain and manufacturer. A traditional egg-based influenza vaccine manufacturing facility at full capacity is capable of processing up to 600K eggs per day. Eggs account for ~50% of bulk vaccine cost. Typical full-scale commercial batches are based on ~250– 350K eggs per day (~1,500–2,000 L of allantoic fluid).
Clarification of Allantoic Fluid
A multiple-step clarification is required for the removal of large contaminants from the allantoic fluid, especially rudimentary tissue compounds such as feather, beak, blood vessel, and blood cells):
- Primary clarification step: A low-speed continuous centrifugation at 4,000–5,000 g is commonly applied during the primary clarification step. Larger particles are separated into the pellet, and the influenza virus stays in the supernatant.
- Secondary clarification step: The secondary clarification step is accomplished through normal flow filtration (NFF). An alternative option is tangential flow filtration (TFF) with a 0.65 µm or 0.45 µm microfiltration membrane device operated with permeate flux control. Because influenza virus is negatively charged at operational pH and can potentially bind to positively charged depth filters containing diatomaceous earth, an optimized high-salt buffer flush, normally as 1-2 M NaCl, can be used to increase process yield.
Virus Inactivation
This chemical treatment step ensures that no active virus (influenza virus or any contaminating virus) proceeds through the downstream process. It is preferably performed as soon as possible in the process to limit the possibility of contamination. However, as inactivation should be performed in a homogenous suspension that is free from particles that may not be penetrated by the inactivating agent, some manufacturers choose to inactivate the virus later in the process. Sometimes, for safety reasons, two inactivation steps are performed. β-propiolactone (BPL), binary ethylenimine (BEI) and formaldehyde are commonly used. As cross-linking agents, they react with viral proteins, eliminating influenza virus infectivity while retaining immunogenicity. Classically, inactivation with BPL is done at 4 °C for 16 hours at a BPL final concentration of 0.1% by volume. (This concentration should not be exceeded at any time during manufacturing.) Formalin is typically used at 0.02% for 18 to 72 hours at 37 °C. 2 mM binary ethyleneimine (BEI) has been proved to inactivate the influenza virus in 48 hours at 37 °C. To prevent aggregation or precipitation from occurring during the inactivation, glycerol and sucrose are often added.
Virus Concentration
At this stage, whole virus particles are concentrated using TFF in order to reduce the volume to be processed in the downstream ultracentrifugation step. Diafiltration also partially removes egg proteins (45 kDa ovalbumin, 76 kDa ovotransferrin, 49 kDa ovoglobulins, 14 kDa lysozyme, and others), DNA, and inactivation agents. Ultrafiltration membranes with 300–1000 kDa can be used in a permeate flux–controlled system; the device and operational parameters must be carefully selected depending on the viscosity and the fouling properties of the influenza fluid. Typical volumetric concentration at this step ranges from 5–40 X. Typical flux is 20–50 LMH at 1.5–3 psi TMP and 5–6 L/min/m² crossflow.
Virus Separation Using Ultracentrifugation
Sucrose density gradient is created by overlaying a lower concentration of sucrose on higher concentrations in a zonal centrifuge chamber. A sucrose gradient could consist of layers extending from a high concentration of sucrose of up to 70% w/v to a low of below 15%. Increments vary depending on the product to be purified. The product fluid is pumped into the centrifuge rotor/ gradient and processed at G-forces in the range of ~40–150K. The particles travel through the gradient of sucrose concentrations until they reach the layer with a density that matches their own. The fraction(s) of interest containing the influenza virus are collected for further downstream processing. The fractions are strain dependant. The virus yield on this step can vary from ~60 to ~90% (The HA assay sensitivity could have ~30% inherent variability.).
Fragmentation of Influenza Virus
Before fragmentation (splitting), the concentrated monovalent (single strain) viral suspension is typically diluted with a sterile buffer (phosphate-buffered saline [PBS], for example) to the standardized optical density (OD) value correlated to the viral protein content in the process fluid.
Fragmentation of viruses is typically executed by adding amphiphilic nonionic detergent such as Triton™ X-100 aqueous solution and/or anionic sodium deoxycholate (DOC) to the suspension of the purified influenza. Polysorbates (Tween® family of products) and cetyltrimethyl ammonium bromide (CTAB) are also suitable for the virus-splitting step.
Fragmentation is normally done with continuous mixing of the process fluid and the detergent(s) for at least one hour at room temperature. The length of the fragmentation step may be extended up to twenty-four hours if necessary. During this step, the viral structure of influenza is dissociated as the envelope is broken down and the internal antigenic components of the virus such as viral RNA-associated capsid nucleoprotein and envelope inner protein matrix (M protein) are released.
Note: The virus inactivation step (in addition to or instead of the one in the upstream part of the process) may be employed on this stage of the process.
Ultrafiltration/Diafiltration
After splitting, the product fluid is subjected to a buffer diafiltration using TFF. This removes the detergent components, and the product is placed in its final buffer. As the virus particles are split, tighter membranes must be used to retain the viral fragments. TFF membranes provide full retention of the viral components of interest while allowing the removal of detergent and sucrose (along with inactivating agents). An average expected flux with 50 kDa is around 30–40 LMH at 5–7 psi TMP and 4–5 L/min/m² crossflow.
Sterile Filtration of Final Purified Bulk
Each separately produced monovalent (single-strain) influenza bulk undergoes a sterile filtration step before it is moved into final formulation and a fill facility. Both PES and PVDF types of sterile-grade membranes may be successfully used in the step. The finished bulk is then formulated, filled, and packaged for administration.
Existing vaccines typically contain 15 mg of each of the three components. The majority of seasonal inactivated split vaccines are not adjuvanted.
The use of squalene-based oil-in-water emulsion-type proprietary adjuvant systems AS03 (Pandemrix®3) and MF59 (Focetria®2) in H1N1 pandemic vaccines enabled the eliciting of protective antibody levels with a lower amount of the viral antigen—7.5 mg per a dose. This so-called dose-sparing effect helps to increase the number of available vaccine doses, which is particularly important in a pandemic when supply cannot meet demand because of limited manufacturing capacity.
Summary: Egg-Based Influenza Vaccine
The manufacturing of egg-based influenza vaccine is an established, well-characterized process with over thirty years of commercial scale application. Even so, it presents numerous unique challenges. The ongoing genetic mutations of the virus require annual production of a seasonal vaccine incorporating components of selected viral strains. Yields of the strains are variable and often could not be known until an actual full-scale production occurs. A vaccine license must be issued for each new seasonal vaccine for the length of one year only.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?