Assay and Identification of Betamethasone Sodium Phosphate by HPTLC
Abstract
This article introduces a highly efficient thin-layer chromatography (HPTLC) method for accurately measuring and identifying betamethasone, a glucocorticoid commonly used in different therapeutic applications. The method utilizes a Silica Gel 60 F254 HPTLC plate and involves two detection approaches, UV light at 254 nm and derivatization with 2,4-dihydroxybenzaldehyde. The method exhibits linearity across a concentration range of 1 to 7 µg/spot. The detection limit (LOD) and quantification limit (LOQ) were established as 0.91 µg/spot and 2.74 µg/spot, respectively. The method was validated with an assay range of 96.0 to 103%, confirming its suitability for quality control of betamethasone in pharmaceutical applications.
Section Overview
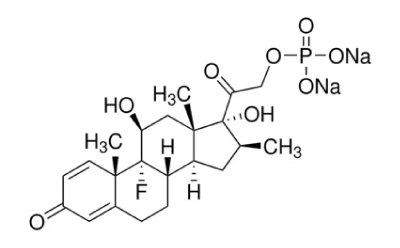
Betamethasone sodium phosphate
Betamethasone Sodium Phosphate is the disodium salt of the 21-phosphate ester of betamethasone, a synthetic glucocorticoid with metabolic, immunosuppressive and anti-inflammatory actions.1
The European Pharmacopoeia monograph for betamethasone sodium phosphate employs high-performance thin layer chromatography (HPTLC) technique for the identification and spectrophotometric method for assay.2,3
Here we show a method for identification and assay of betamethasone sodium phosphate using 2 detection approaches and a densitometric measurement.
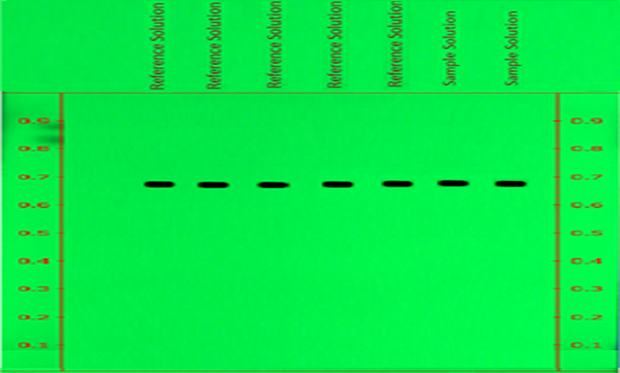
Figure 1.Determination of Repeatability of betamethasone spots for reference solution and sample on chromatogram measured at wavelength 254nm (Detection A).

Figure 2.The principal spot of reference and sample in the chromatogram obtained with the test solution in day light after derivatization (Detection B).
According to Ph. Eur. 11.0, the principal spot in the chromatogram obtained with the test solution should be similar in position, color, and size to the principal spot in the chromatogram obtained with the reference solution. (Figure 2).
Reproducibility
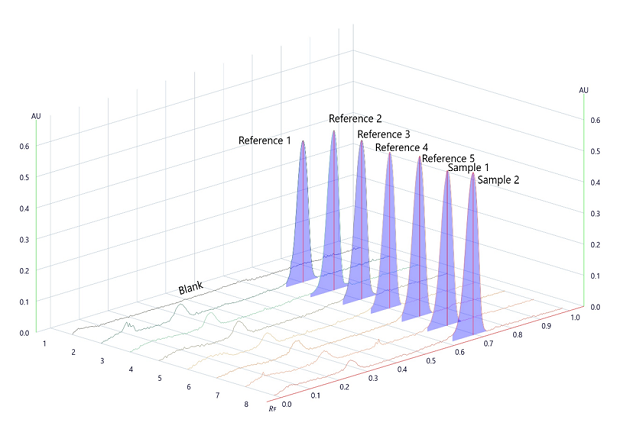
Figure 3.Densitogram for Repeatability and Area under the peak measured at wavelength 254nm.
Assay for Betamethasone Sodium Phosphate by HPTLC
Linearity LOD and LOQ
Linearity of betamethasone sodium phosphate peak area measurements by densitometry at 254 nm for a calibration range of 1 to 7 µg/spot.
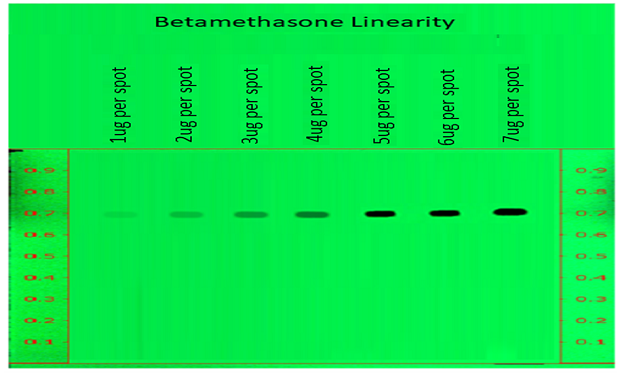
Figure 4.Determination of Linearity of betamethasone sodium phosphate measured at wavelength 254nm.
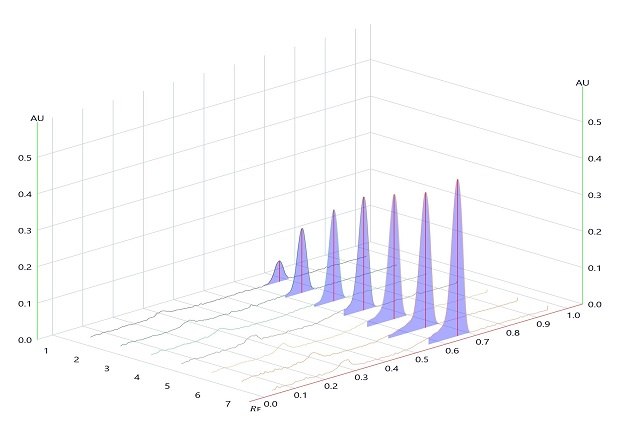
Figure 5.Densitogram for Linearity of betamethasone sodium phosphate measured at wavelength 254 nm.
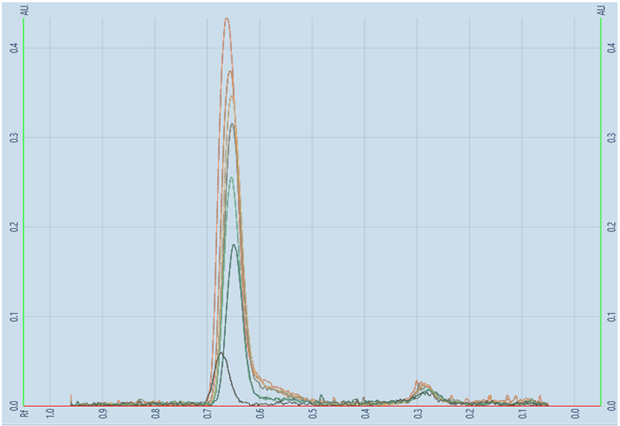
Figure 6.Linearity scan of betamethasone sodium phosphate.
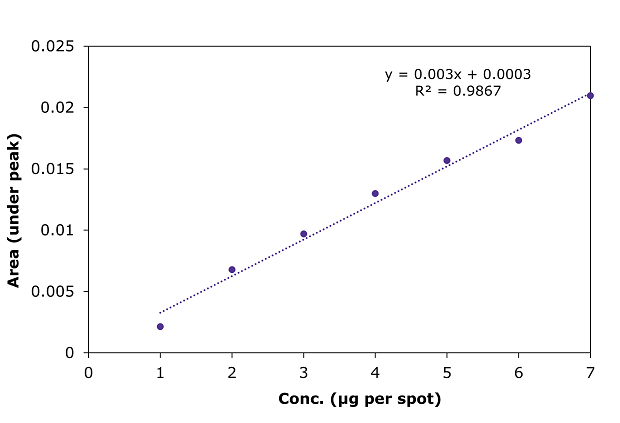
Figure 7.Calibration curve for betamethasone sodium phosphate by HPTLC at 254 nm.
Accuracy and Recovery
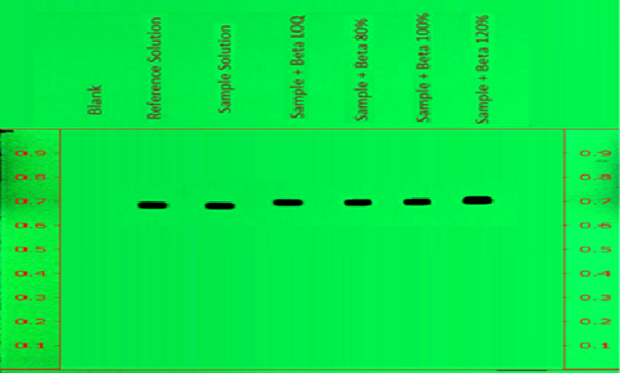
Figure 8.Determination of accuracy of betamethasone sodium phosphate with spiking over sample on chromatogram at measured wavelength 254nm.
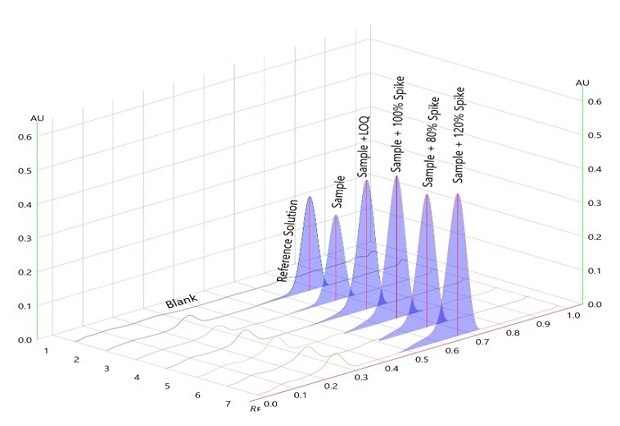
Figure 9.Densitogram for accuracy of betamethasone sodium phosphate with spiking over sample on plate/chromatogram measured at wavelength 254nm.
Conclusion
The presented instrumental HPTLC method is simple, linear, and selective over a concentration range from 1 to 7 µg/spot. The RSD for repeatability of area under the curve and retardation factor is < 2%. The limit of detection (LOD) was 0.91 µg/spot, and the Limit of Quantification (LOQ) was 2.74 µg/spot. The percentage recovery of betamethasone ranged from 96.5 to 104.4%.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?