3D Printed Precision: Selective Laser Sintering for Oral Drug Delivery
Called “the new industrial revolution”, use of three-dimensional (3D) printing has grown substantially in recent years across a wide range of industries.1 Benefits of this approach include a reduction in manufacturing lead times and the ability to meet customer demand more efficiently. This technique is now gaining traction in the pharmaceutical industry.
3D Printing in the Pharmaceutical Industry
Among the applications of 3D printing in the pharmaceutical industry include the ability to produce medications with bespoke properties tailored to the needs of individual patients. 3D printing enables fabrication of personalized dosage forms that can be precisely tailored in terms of shape, size, texture, and release profiles.2 Also known as additive manufacturing because the structure is fabricated layer-by-layer from the bottom up, 3D printing may offer value for solid dosage forms that otherwise would be difficult to produce and customize using conventional approaches.3
Much of the potential of 3D printing stems from the higher degree of flexibility that it offers in manufacturing. With traditional oral solid dose manufacturing like tableting, the final geometry is limited by the machine tooling; as such, creating prototypes can require extensive, complex modifications to the tooling and dedicated formulation development to match target release kinetics. In contrast, additive manufacturing offers maximum flexibility for designing and creating prototypes.
A variety of 3D printing techniques can be leveraged for powder-based, liquid-based, and extrusion-based systems:
- Drop-on-powder (DOP) and selective laser sintering (SLS) can be used for powder-based systems
- Drop-on-drop deposition (DOD) and stereolithography (SLA) can be applied to liquid-based systems
- Fused-deposition modeling (FDM), melt-drop deposition, direct powder extrusion (DPE) or Melt Extrusion Deposition (MED®) are appropriate technologies for extrusion-based systems
This page provides an overview of SLS, describes the advantages of the technique for pharmaceutical applications, and highlights the importance of selecting the appropriate polymers to enable a successful SLS process.
The Selective Laser Sintering Process for Production of Oral Dosage Forms
SLS uses the defined energy of a laser to sinter powder material to create a 3D structure. The sintering process consists of the steps listed below which are repeated until the dosage form is completed and recovered from underneath the powder bed. A schematic of the process is shown in Figure 1.
- A thin layer of powdered material is evenly spread on the building plate.
- The powder bed is pre-heated to optimize the required energy used for sintering.
- A scanning laser beam is directed to draw a specific pattern onto the surface of the powder bed.
- The beam selectively fuses powder particles together and creates the target geometry.
- Once the first layer is completed, a delivery piston and roller distribute a new layer of powder from the powder reservoir on top of the previous one, building the dose layer by layer.
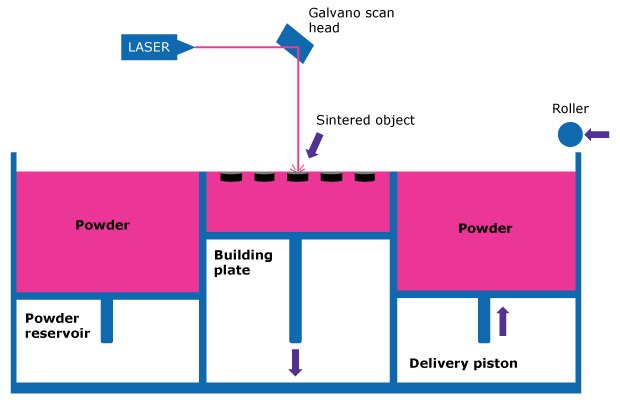
Figure 1.Schematic of the SLS 3D printing process.
Table 1 shows the main processing parameters for SLS which include the laser beam energy and speed, hatching space, particle morphology, powder bed temperature, layer thickness, and laser beam spot size.
In a study to determine the effect of laser speed and power, changes in the laser speed and power were shown to have a significant influence on the absolute mass and relative standard deviation of the mass.7 Moderate laser speed and power resulted in a small mass deviation and thus a uniform output in terms of dimension and mass. If faster production is desired, higher laser speed and power are required.
Advantages of SLS Technology for Production of Oral Dosage Forms
SLS provides the same advantages associated with other 3D printing techniques for pharmaceutical applications including:
- The production of tailored dosage forms for individual patient needs
- Creation of customized shapes that are beneficial for pediatric and geriatric patients
- Solubility enhancement by in situ formation of amorphous solid dispersions
- On-demand manufacturing
- Streamlined drug development due to rapid prototyping, flexible iterations and optimization of the development process
In addition to these advantages common to all 3D techniques, SLS offers unique benefits. For example, while 3D techniques enable production of complex geometries, SLS allows greater creativity and form flexibility. It has been used to create orally disintegrating printlets (ODPs) for visually impaired patients with braille and moon patterns on their surface, enabling patients to identify medications when removed from the original packaging.5 The technique can also be used to create highly porous, rapidly disintegrating structures that can improve patient adherence.2 The porosity of the 3D printed dosage forms, and thus the disintegration kinetics, can be influenced by the SLS laser power and speed.6 In addition, SLS may offer advantages over other 3D printing techniques for manufacturing larger batches of dosage forms (e.g., 30 or 100 tablets per print) due to the instrument’s large print volume and high packing density.2
One consideration related to SLS is that the laser can induce API instabilities/API degradation. However, drug degradation studies have been published that indicate that the effect on drug stability resulting from the laser treatment is not such a great concern as expected.2
Polymer Selection is Critical for Successful SLS
SLS requires the use of thermoplastic polymers as matrices to carry the active pharmaceutical ingredient (API); the laser hardens the polymer, which should be able to absorb the laser light wavelength, by increasing the temperature above its melting temperature. 7
The chemical properties of polymers used in the SLS process such as dynamic viscosity, degree of hydrolyzation, and molecular weight, have a significant impact on drug release and kinetics, as well as the physical properties of the dosage form printed under the same settings.8
A wide range of excipients such as hydroxypropyl methylcellulose (HPMC), microcrystalline cellulose (MCC), povidones, copovidones, crospovidones, polymethacrylates, polyethylene oxide, ethyl cellulose and polyvinyl alcohol can be used to enable immediate and sustained release of the printed dosage form.9 The right polymer will provide the desired balance between printability and product performance, stabilizing the solid state, facilitating drug delivery applications, and enabling broad adoption of this 3D printing technique.10
In a study comparing the results of the different grades of polymers, polyvinyl alcohol (PVA)-based grades, including PVA 4-88 (Parteck® MXP 4-88) and PVA 3-82 (Parteck® MXP 3-82), supported API amorphization, while polyvinylpyrrolidone with vinyl acetate (PVP-VA)-based dosage forms had remaining traces of crystallinity which was reflected in the dissolution results. PVA-based dosage forms showed a rapid release, while the PVP-VA-based dosage forms had a release of only 5%.9
The effect of polymer properties on the dissolution performance has also been evaluated. The findings revealed that the chemical properties of the polymers, such as dynamic viscosity, degree of hydrolyzation, and molecular weight, significantly affect the drug release profile and allow for a modulation of the drug release rate and supersaturation. Additionally, the choice of polymer was shown to influence the physical properties of dosage forms printed using the same settings. Consequently, the chemical properties of the polymer play a crucial role in selecting appropriate manufacturing settings and in shaping the final properties of orally administered dosage forms produced using SLS.9
The lower the viscosity of the polymer, the higher the dissolution rate of the API. Tikhomirov et al. evaluated polymers including PVA 3-82 (Parteck® MXP 3–82) and found that the 82% degree of hydrolysis enabled by PVA-3-82 provided the most suitable ratio of acetate and hydroxyl groups for the enhanced solubility of the lipophilic API.9 This resulted in a supersaturation concentration 40 times higher than the pure API and approximately 2.5 times higher than other PVA grades.
Conclusion
3D printing techniques, including SLS, enable the production of tablets with tailorable properties, expanding the availability of personalized medicines. These techniques are particularly attractive when the manipulation of oral dosage forms is required for certain patient populations including children and for those that suffer from dysphagia. Use of 3D printing can eliminate the need for tools such as tablet splitters or the dispersal of tablets in water prior to administration, both of which can lead to inaccurate dosing and pose a danger to patients.
SLS, in particular, offers many advantages for pharmaceutical production including in the areas of early and rapid prototyping, form flexibility, scalability, and manufacturing flexibility. Tailored release kinetics can be achieved by changing the laser energy input, producing structures of varying porosities, while in situ amorphization supports solubility enhancement.
SLS can be used to manufacture large batches of solid dosage forms from polymers with different physical properties. Careful selection of the polymer that is used in the process is essential as its properties will impact the manufacturing outcome and therapeutic performance. Polymers have an immediate influence on the 3D printing process itself, as well as the properties and stability of the resulting drug product. PVA is an example of a polymer that combines the benefits of a stable amorphous profile and rapid release, both of which offer benefits for SLS.
Collaborating with experienced suppliers of suitable, high-quality polymers and the respective 3D printing technology providers can significantly reduce internal development efforts, especially when tailored formulation support is also available.
Click below to reach us if you need more information about Selective Laser Sintering and one of our experts will contact you.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?