地表水中磷酸盐

磷是生物、植物中的基本元素。1磷在天然无污染水体中以三种形式存在:有机结合磷酸盐、缩聚磷酸盐、正磷酸盐(通常以化学式PO4-P表示)。天然水体中少量存在的磷不会促进植物生长。而当磷浓度上升后,藻类疯长,导致水体富营养化。2, 3
20th世纪中期,化肥、废水、洗涤剂等人造产物导致各种水体因过量的磷污染而广泛呈现富营养化。随后实施的减少磷酸盐浓度的措施,
包括洗涤剂停用磷酸盐和废水处理厂沉淀磷酸盐,使环境中的磷酸盐负荷下降约75%。2, 4
一个启示性例子是对德国康斯坦茨湖(德国博登湖)的磷治理。在1970年代末期,湖中的PO4-P浓度是84 μg/L,而如今主要水体中的PO4-P浓度仅有5-6 μg/L。5, 6
地表水中磷酸盐的分析
分析这么低浓度的PO4-P是有难度的。
按DIN EN ISO 10304-1标准,离子色谱法对磷的检测下限是33 μg/L PO4-P(相当于100 μg/L PO4),比地表水中一般的磷酸盐浓度高很多。光度检测法则灵敏很多。按DIN EN ISO 6878标准,在没有繁复提取步骤的前提下,光度法对磷的检测下限是5 μg/L PO4-P。8
Spectroquant®磷酸盐检测法与DIN EN ISO 6878标准光度法的比较
两种方法的检测原理是一样的。即正磷酸盐在硫酸盐溶液中,与钼酸盐和锑离子反应生成磷钼酸,该化合物被抗坏血酸还原生成磷钼蓝(PMB),光度法测定含量。9除了预设校准曲线外,Spectroquant®检测试剂盒与标准方法相比的另一优势是使用比较方便。按DIN EN ISO 6878方法检测时,需要分开配制各种试剂。至少要配制5种试剂,还不包括校准程序需要的标准溶液。8
Spectroquant®检测试剂盒为检测专门提供配好的即用型试剂。当在100-mm比色皿中灵敏测定磷酸盐时,实验人员只需要加入2种试剂。对于20 mL样品,仅需加入20滴PO4-1试剂和4平勺PO4-2试剂(使用配送的微量勺)。反应5分钟后,在光度仪中测量样品的显色强度,最后可直接在仪器显示屏上读取磷酸盐浓度。详情参见Spectroquant®磷酸盐检测试剂盒(货号114848)在线产品页面上的应用说明——《地下水和地表水中正磷酸盐的灵敏检测》。10
为了证明Spectroquant®检测试剂盒测定地表水中磷酸盐浓度的适用性,使用试剂盒测定6种水样中的PO4-P含量。作为比较,同时进行DIN EN ISO 6878方法的对照分析。按DIN 32645测定DIN法的检出限为
0.003 mg/L PO4-P。
两种方法的结果相当:在6种水样中,4种的PO4-P浓度低于检测范围,只有2种水样的检测值在检测范围内。在布罗姆巴赫湖水样中,Spectroquant和DIN法的检测结果相差0.0031 mg/L PO4-P。在阿尔特米尔湖水样中,检测结果仅相差0.0004 mg/L PO4-P,Spectroquant®相对于DIN EN ISO法的回收率达97%。
除了对照分析以外,还采用加标法检测样品。检测中,在5种湖水样品和1种水库样品中加入4种不同浓度的PO4-P标准品,测定每种样品的回收率。图1为检测结果。

图 1.加入标准品后回收的正磷酸盐浓度
在所有样品中,加入的标准品浓度均可回收。平均加标回收率为105% ± 5%。
如要进一步增强准确度,可进行目标检测范围的用户校准。在检测范围
0.0025 – 0.0250 mg/L PO4-P内,绘制10点校准曲线。曲线图见图2。
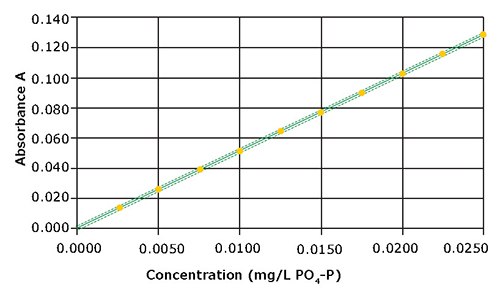
图 2.0.0025 – 0.0250 mg/L检测范围内的校准曲线
该方法呈现良好的线性关系。与预设校准相比,检测性能大幅提高,方法标准偏差±0.0001 mg/L,方法变异系数±0.88%,置信区间±0.0003 mg/L(表2)。
总结
Spectroquant®磷酸盐检测试剂盒(产品编号1.14848)是测定地下水和地表水中正磷酸盐的快速、实惠、精准的标准替代方法。检测结果与DIN EN ISO 6878方法相当。已通过加标法证明该检测试剂盒适于定量分析地下水和地表水中的PO4-P。
产品列表
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?