Trost Ligands for Allylic Alkylation
The Tsuji Trost cycle is a synthetic methodology used in organic chemistry for the construction of complex molecules. It involves the use of a palladium-catalyzed reaction to facilitate the formation of carbon-carbon bonds through the coupling of allylic alcohols with nucleophiles. Palladium-catalyzed asymmetric allylic alkylation (AAA) is a powerful method for the efficient construction of stereogenic centers. In contrast to many other catalytic methods, AAA has the ability to form multiple types of bonds (C–C, C–O, C–S, C–N) with a single catalyst system.
Section Overview
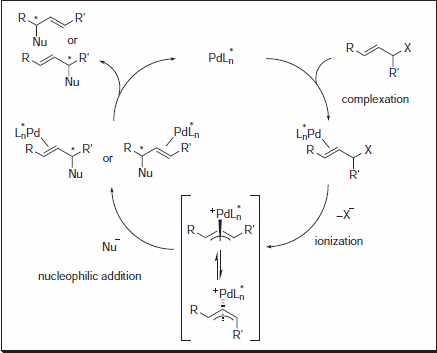
Figure 1.Nucleophilic Addition
The Trost group at Stanford University has pioneered the use of C-2 symmetric diaminocyclohexyl (DACH) ligands in AAA, allowing for the rapid synthesis of a diverse range of chiral products with a limited number of chemical transformations. Reactions are typically high-yielding, and excellent levels of enantioselectivity are observed.1
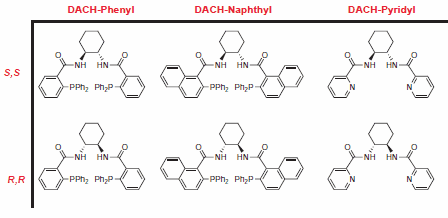
Figure 2.Trost Ligands
Carbon Nucleophiles
In early examples of this methodology, Trost and co-workers demonstrated diesters are competent nucleophiles for the deracemization of cyclic allylic acetates, to afford chiral malonate derivatives. Since that time, soft carbon nucleophiles such as barbituric acid derivatives, β-keto esters, nitro compounds, and many others have been employed in AAA for assembly of tertiary and quaternary asymmetric centers.
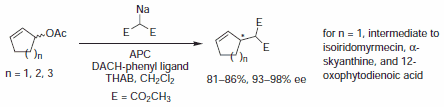
Figure 3.Malonate Nucleophiles
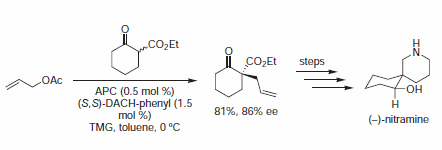
Figure 4.β-Keto Ester Nucleophiles
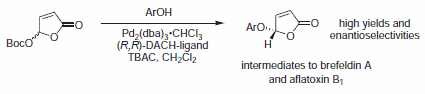
Figure 5.Alcohol Nucleophiles
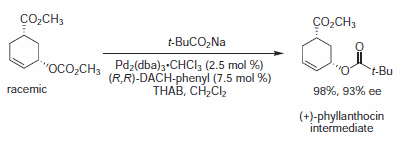
Figure 6.Carboxylate Nucleophiles
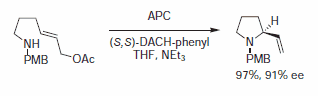
Figure 7.Alkylamines Nucleophiles
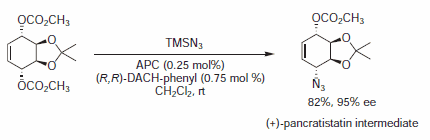
Figure 8.Azide Nucleophiles
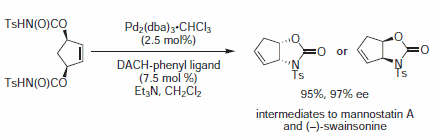
Figure 9.Imide Nucleophiles
Molybdenum-Catalyzed Reactions
The mechanism of the molybdenum-catalyzed AAA reaction is presumed to be distinctly different from the analogous Pd-catalyzed reaction, and in some cases, the levels of regio-, enantio-, and diastereoselectivity are enhanced relative to the palladium-catalyzed reaction. Trost and Dogra report the total synthesis of (–)-Δ9-trans-tetrahydrocannabinol, a psychomimetic of marijuana, utilizing a molybdenum catalyst.9 An overall yield of 30% of enantiomerically pure (–)-Δ9-trans-tetrahydrocannabinol (Figure 10).
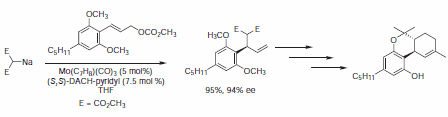
Figure 10.Molybdenum-Catalyzed Reactions
References
如要继续阅读,请登录或创建帐户。
暂无帐户?