Palladacycle Coupling Catalysts
Bedford Catalysts
Palladacyclic catalysts developed by Bedford’s group are examples of a select group of catalysts capable of affecting a variety of coupling reactions using difficult to activate aryl chlorides at very low catalyst loadings (Figure 1).1 For example, monomeric complex 1, either as an isolated species or from the in situ treatment of 2 with PCy3, exhibits high catalytic activity in the Suzuki coupling reaction of arylboronic acids and aryl chlorides, with turnover numbers (TONs) exceeding 1,000,000!
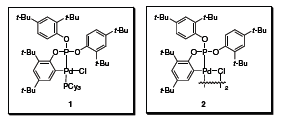
Figure 1
As shown in Table 1, both electronically activated and unactivated aryl chlorides couple with simple boronic acids at catalyst loadings as low as 0.00005%. The optimized protocol calls for two equivalents of phosphine ligand relative to 2, and the use of Cs2CO3 as a base.
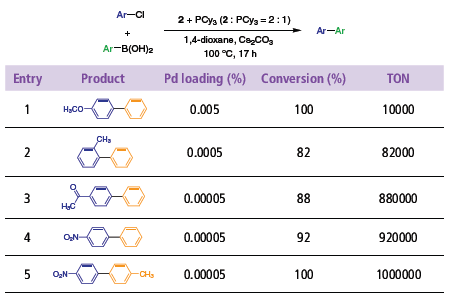
Table 1
Reaction conditions nearly identical to those applied to the Suzuki coupling of aryl chlorides can also be applied to the Stille coupling of aryl- or vinyltin reagents with aryl chlorides to give functionalized biaryls or styrene derivatives (Scheme 1).2
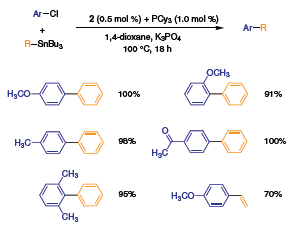
Scheme 1
Lastly, the treatment of precatalyst 2 with P(t-Bu)3 generates a catalyst system that is highly active in the amination of aryl chlorides with diarylamines, alkylarylamines, and dialkylamines (Scheme 2).3 Yields typically exceed 80%, and as in other coupling reactions, catalyst loadings are low (1 mol %) relative to the more commonly used amination protocols.
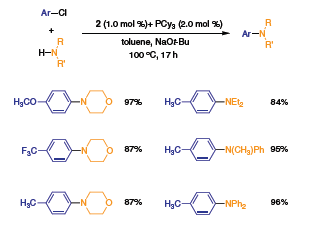
Scheme 2
Nájera Catalysts
Oxime-derived palladacycles 3 and 4 (Figure 2) introduced by Professor Carmen Nájera and co-workers at the Universidad de Alicante (Alicante, Spain) are very efficient phosphanefree precatalysts for a variety of carbon–carbon bond forming processes.4 They are thermally robust and insensitive to oxygen and moisture, slowly forming nanoparticulate palladium under the reaction conditions.5
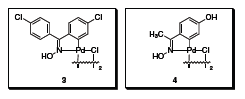
Figure 2
Catalyst 3 provided the highest activities in carbon–carbon bond forming processes carried out in organic solvents, while catalyst 4 proved to be more active in water or aqueous solvents.4 Examples of the carbon–carbon forming process mediated by these palladacycles include the Heck-Mizoroki,6 Suzuki-Miyaura,7,8 Sonogashira,9 Sila-Sonogashira,9 Ullmann, and Stille10 coupling reactions.
Palladacycle 4 is an efficient precatalyst for mono- and β,β- diarylation of electron deficient olefins (Scheme 4),5 while 3 effectively catalyzes the homocoupling of terminal acetylenes in a direct synthesis of conjugated diynes (Scheme 5).9
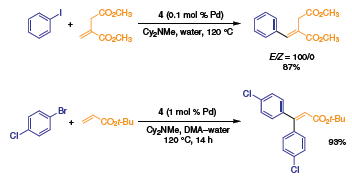
Scheme 4

Scheme 5
Palladacycle 1 also promotes the copper-free synthesis of ynones11 and the direct synthesis of 2,3-disubstituted indoles6 from terminal and internal acetylenes (Scheme 6).
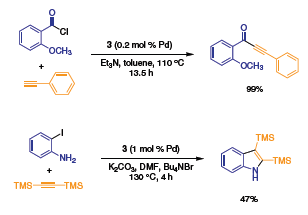
Scheme 6
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?