Nok – Third-Generation Amphiphile for Cross-Coupling Chemistry in Water
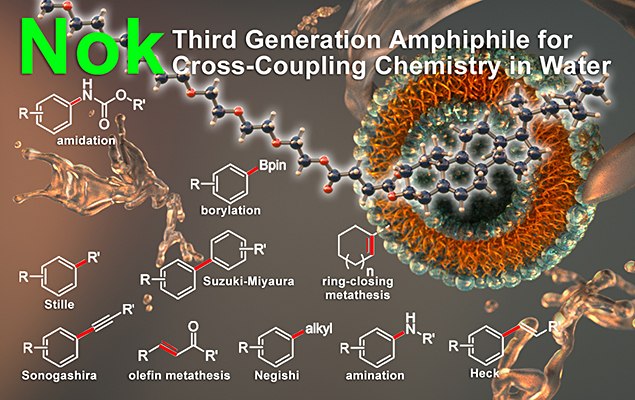
Introduction
Micellar catalysis has made many of the most synthetically useful transformations possible under aqueous conditions, vastly reducing the impact of organic solvent on reaction waste streams. This technology is enabled by the aggregation of amphiphiles into nanomicelles, which can surround organic matter and provide a facile environment for transformation to take place. Professor Bruce Lipshutz’s group has shown this concept to be highly effective with previous amphiphiles such as polyoxyethanyl-α-tocopheryl sebacate (PTS, 698717) and DL-α-tocopherol methoxypolyethylene glycol succinate (TPGS-750-M, 733857). While both of these designer surfactants have been shown to be highly effective in mediating several organic reactions in water and at room temperature, the potential for even more cost-effective amphiphiles is still a viable discovery. Recently, the Lipshutz group reported a new amphiphile, β-sitosterol methoxyethyleneglycol succinate (SPGS-550-M, 776033) otherwise known as Nok.1 The third-generation surfactant, Nok, is the newest addition to our designer surfactant portfolio and is the most cost-effective option to date.
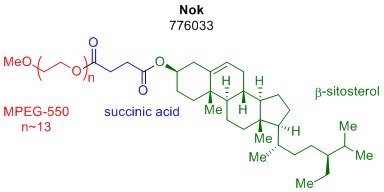
Advantages
In addition to its low cost, Nok displays comparable, if not better, conversions and isolated yields to the first two generations of designer surfactants (PTS and TPGS). More importantly, like the rest of the portfolio, Nok provides potential in improving the environmental profile of the process used to carry out the several transformations that can be implemented under the aqueous medium. The Lipshutz group reported an improvement in regards to a commonly used measure to determine organic waste produced, E Factor.2 Due to the recyclability of the water medium and minimal solvent required to extract organic matter from the vessel, their group was able to show an improvement in waste production in the Suzuki-Miyaura coupling of 2-bromobenzotrifluoride and 6-methoxy-2-naphthaleneboronic acid.1
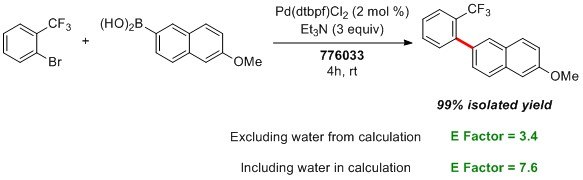
Since the reaction can be run at very high reactant concentration within the Nok micelles, little water is needed, which is why the E Factor did not increase significantly when water was included in the calculation (3.4 vs 7.6).1
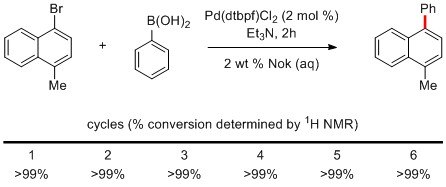
Like reactions run in PTS and TPGS, the water layer can be reused several times for the same reaction. For example, the Suzuki-Miyaura coupling between 1-bromo-4-methylnaphthalene and phenylboronic acid can be recycled six times without erosion of conversion.1
Representative Applications
The following reactions can be carried out using Nok at room temperature:
- Suzuki–Miyaura Cross-Coupling
- Negishi Cross-Coupling
- Olefin Metathesis
- Miyaura Borylation
- Sonagashira Cross-Coupling
- Heck Reaction
- Stille Cross-Coupling
- Buchwald–Hartwig Aminations
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?